All published articles of this journal are available on ScienceDirect.
A Novel approach for Corneal Remodeling of Laser Asymmetric Keratectomy with Collagen Cross Linking in Patients with Keratoconus Suspect
Abstract
Background
True Keratoconus Suspect (KCS) is an absolute contraindication to LASIK or Photorefractive Keratectomy (PRK) due to postoperative ectasia.
Objective
To evaluate the effectiveness of laser asymmetric keratectomy with collagen cross-linking (L-LAK-CXL) in myopic patients with suspected keratoconus (KCS).
Methods
This study included 40–44-year-old four myopic (-2.50 to -5.50 D) patients (4 eyes), of KCS with focal corneal steepening over +47.0 D and peripheral asymmetric corneal thickness. L-LAK-CXL was performed for both original ablation of refractive errors and crescentic customized ablation of the thicker peripheral cornea selectively and myopic changes due to the ablation of the peripheral thicker cornea simultaneously, followed by CXL without the epithelium. We compared preoperative and postoperative ocular findings, including corneal symmetry (total differences of the corneal thickness in four directions (SUM) and decentration of the thinnest point (DISTANCE)) and tear break-out time (TBUT).
Results
From preoperative to postoperative, spherical equivalent (D, average) decreased from -3.38 to -0.34, uncorrected distance visual acuity (LogMAR) increased from 0.53 to 0.00, and Kmax (average D) had decreased from +48.3 to +43.95, central pachymetry (CP, µm, average) decreased from 574 to 511. Postoperative corneal symmetry increased markedly owing to decreased SUM and DISTANCE scores. TBUT increased over 15 s postoperatively. No post-operative corneal ectasia was observed.
Conclusion
L-LAK-CXL improved corneal symmetry in myopic patients with KCS by reducing SUM, decreasing DISTANCE and Kmax, increasing TBUT, and demonstrating good postoperative visual outcomes.
1. INTRODUCTION
Keratoconus (KC) is a dome-shaped front of the eye that becomes thinner and gradually bulges outward into a conical shape, which results in blurred vision and may cause sensitivity to light and glare [1].
Although keratoconus suspect (KCS) shows focal steepening, maybe over +47.0 D or slight bowing of the posterior corneal surface on elevation topography, it does not result in visual impairment. To date, KCS has not been specifically defined, as there are several definitions [1-9].
A topographic KCS will have a focal corneal steepening over +47.0 D and may show an asymmetrical, truncated, or skewed-axis bowtie [2, 3]. Waring [4] identified KCS as “the fellow eyes of unilateral KC that had no slit lamp findings” overlapped forme fruste keratoconus. The other view of Li al [6]. defined the fellow eye independently as “no slit lamp finding, no scissoring on retinoscopy, and asymmetric bowtie pattern with a skewed radial axis on videokeratography only. Some authors [7-9] provided their definitions of KCS, which include the following: no clinical sign of KC, minor topographic asymmetry, steep corneal curvature more than +47.0 D, central corneal thickness less than 500 µm, and oblique astigmatism more than 1.5 D.
True KCS is an absolute contraindication to LASIK or photorefractive keratectomy (PRK) due to postoperative ectasia [10-14]. PRK in eyes with KCS may be safe and effective for the treatment of myopia and astigmatism in carefully selected patients [15-17].
Among these findings of KCS, focal steepening over +47.0 D and peripheral corneal asymmetry of the thickness could be very important because of the possibility of post-LASIK or PRK corneal ectasia. In myopic patients with topographic corneal thickness asymmetry and steep curvature of the cornea more than +47.0 D, LASIK or PRK cannot avoid postoperative corneal ectatic changes, possibly corneal biomechanical interaction of corneal thickness, corneal stiffness, and IOP, causing corneal morphological changes in the thin region of the cornea in optical aberrations [18-20]. The introduction of eccentric thinning is required to produce an asymmetric displacement and an eccentric peak in the dioptric curvature value when a normal elastic modulus is used [16]. Recently, laser asymmetric keratectomy (LAK) as a biomechanical customization method, which was integrated with Vision Up software (Well C, Busan, South Korea) and was approved by the Republic of Korean Food and Drug Administration (FDA) in 2021, has shown good results. Moreover, the cornea is symmetric in myopic patients with the peripheral asymmetric thickness of the cornea because it could ablate only the thicker peripheral cornea selectively as a customization method [21-26].
For collagen cross-linking, riboflavin (vitamin B2) was administered in conjunction with ultraviolet A light (UVA, 365 nm). The interaction between riboflavin and UVA causes the formation of reactive oxygen species, leading to the formation of additional covalent bonds between collagen molecules, resulting in biomechanical stiffening of the cornea [27-29].
This study aimed to report the initial outcomes of laser asymmetric keratectomy with collagen cross-linking (L-LAK-CXL) to correct the original and customization ablation with LASEK combined with LAK(L-LAK) and maintain long-term stability with CXL in myopic patients with KC, accompanied by peripheral corneal thickness asymmetry (a SUM of ≥80 µm) and focal corneal steepening (Kmax) over + 47.0 D.
2. CASE PRESENTATION
This retrospective study included four myopic patients (four eyes) with KCS who underwent L-LAK-CXL at Woori Eye Clinic between September 2021 and January 2022. A KCS was diagnosed when there is steep keratometric curvature greater than 47 D on topography or a bowtie pattern asymmetrically, a normal cornea on slit lamp 4biomicroscope, and at least 1 of the following signs: minor topographic asymmetry oblique cylinder greater than 1.50 D, central corneal thickness less than 500 µm, or clinical KC in the fellow eye [2-8].
A KCS who underwent L-LAK-CXL, those with over -1.50 diopter in refraction, those manifesting both steep keratometric curvature greater than 47 D (Kmax) > +47.0 D and minor topographic asymmetry, those with 20/20 in corrected vision, and those with postoperative follow-up examination over 12 months were included in this study. Patients with follow-up examinations in < 12 months and a history of other corneal conditions, including corneal problems, were excluded. Pre- and post-LAK-CXL results were analyzed. This study was approved by the Korean National Institute for Bioethics Policy (approval No P01- 202303 -01- 010). Written informed consent was obtained from all patients.
L-LAK was performed by the same doctor (BMM) using an excimer laser (Kera Harvest Inc., Taiwan) [21-26] for all patients. The 6.0 – 6.5 mm optic zone and 6.5-8.5 mm in transitional zone were ablated for correcting refractive errors without epithelium of a 9.0–9.5-mm in diameter for LASEK under topical anesthesia of 0.50% propacaine hydrochloride (Alcaine, Alcon NV, Vilvoorde, Belgium). To perform corneal remodeling with LAK [21-26], we used Vision-Up software (Well C, Busan, Republic of Korea) to analyze the corneal deviations based on Orbscan II (Bausch & Lomb, Bridgewater, NJ, USA) preoperative corneal maps (Fig. 1). With this, we calculated the amount of ablation on both the thicker peripheral cornea and the center cornea due to a myopic shift (crescentic customized ablation, Fig. 2a), as determined by LAK. We performed the original ablation (Fig. 2b) for original refractive errors and crescentic customized ablation for both the thicker peripheral cornea and the central cornea to correct the myopic shift due to LAK (total ablation, Fig. 2c) simultaneously with the same laser machine; therefore, we were able to ablate the cornea symmetrically without changing the refractive power (Figs. 3 and 4).
CXL [27-29] was performed after L-LAK in the epithelium-off state. A 0.1% Riboflavin (10 mg riboflavin-5-phosphate in 10 mL dextran-T-500 20% solution) (Vibe X; Avedro Inc., Waltham, MA, USA) was topically administered every 5 min for 30 min. Before UV irradiation, the surgeon confirmed that riboflavin was fully absorbed into the corneal stroma by slit-lamp inspection and the presence of a riboflavin flare in the anterior chamber. The cornea was exposed to UV light at a wavelength of 370 nm and an irradiance of 3.0 mW/cm2 of UVA light (Avedro Inc., Boston, MA, USA) for 30 min, followed by T-lens fitting. Topical antibiotics were administered every 6 h for one week, and a steroid eye solution was administered every 6 h for six weeks.
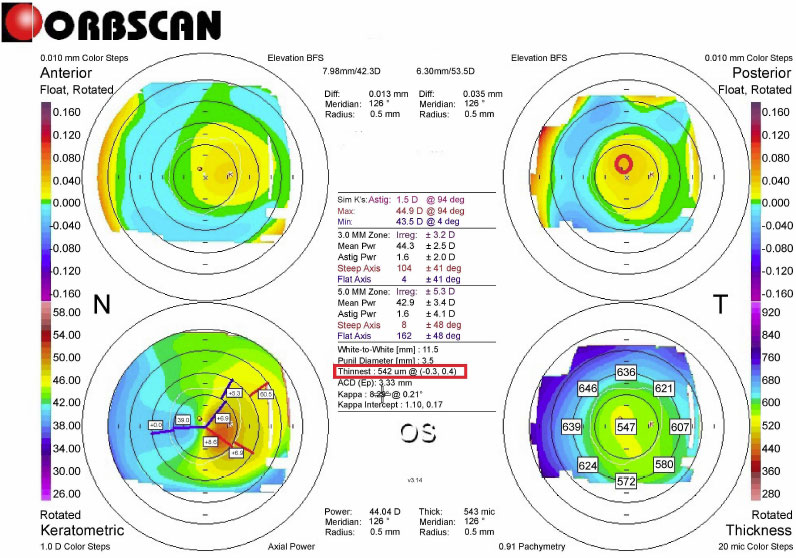
Pre-L-LAK-CXL Topography of Case 1. SUM: 165 µm, Distance (mm): 0.5. Kmax is 48.6 D.
The following findings during the pre- and postoperative L-LAK-CXL (from 12 to 18 months) were analyzed; uncorrected distance visual acuity (UDVA) (LogMAR), spherical equivalent (SE), sphere, cylinder, Kmean, Kmax, IOP, central pachymetry (CP), thinnest pachymetry (TP), pupil size, SUM (µm), DISTANCE (mm), tear break out time (TBUT) (second), tear osmolarity (mOsmol/L) were noted. An auto-refractometer/keratometer was used and converted to SE. The UDVA at a distance of 6 m was converted into the logarithm of the minimum angle of resolution (LogMAR). The IOPs were measured using an applanation tonometer. CP, TP, and pupil size were measured using the Orbscan II. Preoperative and postoperative corneal symmetries were evaluated using SUM and DISTANCE on OrbScan maps (Figs. 1 and 3) [21-26]. TBUT and tear osmolarity were measured using a Keratograph 4 (Oculus, Germany).
3. RESULTS
The patients aged 40–44 years (average: 42.5 years). There were three male and 1 female patients (Table 1). Intraoperatively, within the 6.4 mm optic zone, ablation depths (µm) of the central optic zones and peripheral cornea on the thicker area were 45 –96 (average: 69.3) and 50 – 76 (average: 64), respectively due to L-LAK. Moreover, myopic changes (in diopters) due to LAK were -1.25 – -1.75 (average: 1.5). Furthermore, the residual stromal depth (µm) was 392–486 (average: 444) due to L-LAK. The follow-up period was 12–18 months (mean, 16 months) (Table 1).
The UDVA (LogMAR) improved from 0.53 to 0.00. Hence, L-LAK-CXL resulted in good outcomes without cylindrical axial changes. Additionally, the IOPs and pupil

| Case Number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age (year) | 43 | 40 | 43 | 44 |
| Gender | F | M | M | M |
| Right (R) / Left (L) | L | R | R | R |
| Follow up (months) | 12 | 18 | 16 | 18 |
| Intraoperative findings | - | - | - | - |
| Optic zone (mm) | 6.4 | 6.4 | 6.4 | 6.4 |
| Ablation depth (µm) | - | - | - | - |
| Central cornea | 96 | 46 | 90 | 45 |
| Peripheral asymmetric ablation | 76 | 70 | 60 | 50 |
| Residual stromal depth (µm) | 392 | 486 | 440 | 456 |
| Myopic change due to LAK (D) | -1.50 | -1.75 | -1.50 | -1.25 |
| Items | Preoperative | Postoperative (months) | ||||||
|---|---|---|---|---|---|---|---|---|
| Case number | 1 | 2 | 3 | 4 | 1 (12) | 2 (18) | 3 (16) | 4 (18) |
| SE (D) | -5.50 | -1.75 | -3.75 | -2.50 | -0.50 | 0 | -0.37 | -0.50 |
| Sphere (D) | -5.0 | -1.0 | -2.25 | -2.50 | -0.50 | 0 | 0 | -0.50 |
| Cylinder (D) | -1.0 | -0.50 | -3.0 | 0 | 0 | 0 | -0.75 | 0 |
| UDVA (LogMAR) | 1 | 0.8 | 0.1 | 0.2 | 0 | 0 | 0 | 0 |
| IOP (mmHg) | 14 | 11 | 16 | 13 | 14 | 16 | 15 | 14 |
| Pupil size (mm) | 5.7 | 4.7 | 4.8 | 3.6 | 5.6 | 4.6 | 4.7 | 3.6 |


| Items | Preoperative | Postoperative (months) | ||||||
|---|---|---|---|---|---|---|---|---|
| Case number | 1 | 2 | 3 | 4 | 1(12) | 2 (18) | 3 (16) | 4 (18) |
| Keratometry (D) | - | - | - | - | - | - | - | - |
| Mean K | 43.75 | 46.00 | 45.88 | 44.30 | 39.0 | 43.20 | 44.8 | 42.00 |
| Kmax | 48.60 | 48.40 | 48.20 | 48.00 | 40.90 | 45.20 | 45.20 | 44.50 |
| Pachymetry | - | - | - | - | - | - | - | - |
| CP (µm) | 547 | 591 | 594 | 562 | 451 | 559 | 501 | 534 |
| TP (µm) | 542 | 580 | 580 | 541 | 446 | 555 | 496 | 519 |
| CP-TP (µm) | 5 | 11 | 14 | 21 | 5 | 4 | 5 | 15 |
size of the patients were similar pre- and post-operatively (Table 2). Focal corneal steepening (Kmax) (in diopters on average) had decreased from +48.30 to +43.95. The CP, TP, and CP-TP (in µm on average) had also decreased from an average of 574, 561, 13 to 511, 504, 7, respectively (Table 3). No corneal ectatic changes were observed after undergoing L-LAK-CXL.
Furthermore, the SUM and DISTANCE had decreased (from 172 to 71 µm on average, from 0.85 to 0.29 mm on average, respectively) due to pre-to post- L-LAK-CXL (Table 4). The TBUT (in seconds on average) markedly increased from 5.14 preoperatively to 19.44 post-L-LAK-
| Items | Preoperative | Postoperative (months) | ||||||
|---|---|---|---|---|---|---|---|---|
| Case number | 1 | 2 | 3 | 4 | 1(12) | 2 (18) | 3 (16) | 4 (18) |
| SUM (µm) | 165 | 207 | 158 | 158 | 86 | 63 | 68 | 66 |
| DISTANCE (mm) | 0.5 | 0.78 | 0.89 | 1.21 | 0.41 | 0.1 | 0.43 | 0.21 |
| Items | Preoperative | Postoperative (months) | ||||||
|---|---|---|---|---|---|---|---|---|
| Case | 1 | 2 | 3 | 4 | 1(12) | 2 (18) | 3 (16) | 4 (18) |
| TBUT (second) | 5.78 | 3.96 | 3.70 | 7.11 | 14.66 | 21.98 | 23.73 | 17.38 |
| Tear osmolarity (mOsmol/L) | 0.76 | 0.46 | 0.41 | 0.61 | 0.13 | 0.24 | 0.33 | 0.12 |
CXL. However, the tear osmolarity (average, mOsm/L) decreased from 0.56 preoperatively to 0.21 post-L-LAK-CXL (Table 5).
4. DISCUSSION
All four patients with KCS showed steep keratometric curvature greater than 47 D (from +48.0 to +48,60 D). The thinnest pachymetry were 542, 580, 580, and 541 μm in the four patients, respectively, much higher than the 500-μm criteria for identifying KCS. For focal corneal steepening (Kmax), the postoperative Kmax was flattened to +43.95 D compared to that of the pre-L-LAK-CXL (+48.30 D on average).
However, the most important findings were the SUM values: the sum of corneal thickness differences in four directions on the Orbcan pachymetric map. The higher the SUM was, the more the postoperative thinner cornea could be moved forward owing to the corneal biomechanical interaction of corneal thickness, corneal stiffness, and IOP, resulting in optical aberrations.
In these four patients, the SUM ranged between 158 and 207 μm, which was higher than that of the symmetric cornea.
The SUM on the Orbscan map and focal corneal steepening (Kmax) markedly decreased postoperatively. Moreover, SE and UDVA showed good outcomes without corneal ectatic changes after L-LAK in any of the four patients. In cases of the SUM >80 µm, corneal biomechanical interaction of corneal thickness, corneal stiffness, and intraocular pressure could cause the thinner corneal region to protrude, leading to ectasia [21, 26, 30-36]. The asymmetric biomechanical property distribution of asymmetric corneal thickness causes curvature changes [30-36]. The SUM markedly decreased from 172 µm preoperatively to 71 µm (on average) after L-LAK-CXL, implying that the above parameters might be markedly decreased with L-LAK. Moreover, the reduction in DISTANCE between the visual axis center and the thinnest point was changed from 0.85 mm to 0.29 mm on average after L-LAK-CXL. This indicated an improvement in corneal symmetry. Therefore, we were able to correct refractive errors in myopic patients with KC without postoperative corneal ectasia.
In this study, the topography was assessed using an Orbscan map, which can easily calculate the DISTANCE [21-26]. Scheimpflug corneal topography and epithelial mapping using corneal OCT are currently used [37, 38].
Moreover, TBUT (in seconds on average) had markedly increased from 5.14 at pre-L-LAK to over 15 (19.44) at post-L-LAK-CXL. It may be expected that focal steepening of the cornea pre-L-LAK-CXL and large CP-TP could make the tear break easily. However, flattening of the steepening cornea and smaller CP-TP postoperatively could keep the tear layer intact [39-42].
Vision-Up software (Well C, Busan, Republic of Korea) was accurate and useful for calculating the thicker peripheral cornea and the amount of myopic shift due to LAK. Therefore, we could correct the original ablation (Fig. 2b) of refractive errors and crescentic customized ablation (Fig. 2a) of the thicker peripheral cornea and myopic shift due to peripheral corneal ablation simultaneously. As a result, we could create a symmetric cornea with minimal refractive errors in myopic patients with KC (Figs. 3 and 4) [21-26]. LAK reduced the SUM, made the cornea to be symmetric, and resolved shortcomings of laser refractive surgery (LASIK or LASK). Moreover, LASIK or LASEK combined with LAK (L-LAK) could produce better surgical outcomes in myopic patients with asymmetric corneal thickness (SUM Orbscan maps >80 µm) [25, 26]. LAK could also be a new customization method [25, 26] for achieving symmetric cornea in patients with KCS without postoperative corneal ectasia. Additionally, CXL had well-maintained stabilization [27-29].
We could avoid the adverse effects due to asymmetric cornea in cases of a SUM (≥80 µm) with corneal remodeling using LAK to make the cornea symmetric [21-26]. Thus, it would be safe to correct myopic patients with KCS, particularly in those with asymmetric cornea (SUM >80 µm) and focal steepening over +47.0 D. L-LAK with or without CXL could be an innovative idea to 1) avoid the adverse effects of laser refractive surgery [21, 22, 25, 26], 2) develop a novel approach to enhancement in patients with myopic regression after LASIK/LASEK [23, 24, 42], and 3) reduce the protrusion on the thinner portions of the cornea in patients with early KC or KCS with peripheral asymmetry of the corneal thickness [21-26]. Further studies on L-LAK-CXL are required.
This was a pilot study, and therefore has several limitations. KCS is an absolute contraindication to LASIK or photorefractive keratectomy (PRK) due to postoperative ectasia [10-14], especially in cases of KCS with a SUM (≥80 µm). A comparative study on the differences between L-LAK-CXL and traditional operative treatments (LASIK or PRK) of KCS could not be performed. Further studies with more patients with KC and longer follow-ups should be conducted in the future.
CONCLUSION
L-LAK-CXL, a novel corneal remodeling technique, resulted in decreased SUM and DISTANCE, increased corneal symmetry, and increased TBUT, with good visual outcomes in myopic patients with KC suspected of manifesting peripheral asymmetry of corneal thickness and focal corneal steepening over + 47.0 D.
LIST OF ABBREVIATIONS
| LogMAR | = The logarithm of the minimum angle of resolution |
| LAK | = Laser asymmetric keratectomy |
| D | = Diopters |
| F | = Female |
| M | = Male |
| SE | = Spherical equivalent |
| UDVA | = Uncorrected distance visual acuity |
| IOP | = Intraocular pressure |
| CP | = Central pachymetry |
| TP | = Thinnest pachymetry |
| CP-TP | = Subtraction of two pachymetry values. |
| TBUT | = Tear break out time |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all participants involved in the study for the publication of details of their medical care and any accompanying images.
STANDARDS OF REPORTING
CARE guidelines were followed.
AVAILABILITY OF DATA AND MATERIAL
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors confirm that there is no conflict of interest related to the manuscript.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.co.kr) for English language editing and Rag Seon Han, Eun Mi Jang, Ji Yeon Choi, Sun Hee Lee, Mi Kyung Kim, Ji Suk Kwon, Hye Won Jung, and Kyoung Mi Ryu at the Woori Eye Clinic for their assistance with the ocular examinations.


