RESEARCH ARTICLE
Bilateral Choroidal Metastases from Endobronchial Carcinoid Treated with Somatostatin Analogues
Deborah De Bruyn1, *, Jan Lamont2, Erik Vanderstraeten3, Simon Van Belle4, Elise Platteau1, 5, Julie De Zaeytijd1, Kristien P. Hoornaert1, 5
Article Information
Identifiers and Pagination:
Year: 2016Volume: 10
First Page: 160
Last Page: 165
Publisher ID: TOOPHTJ-10-160
DOI: 10.2174/1874364101610010160
Article History:
Received Date: 27/1/2016Revision Received Date: 13/6/2016
Acceptance Date: 21/8/2016
Electronic publication date: 30/09/2016
Collection year: 2016

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Objective:
To describe a patient with bilateral multifocal choroidal metastases from an endobronchial carcinoid treated with a somatostatin analogue.
Method:
A 60-year-old woman presenting with photopsia in the left eye underwent an extensive ophthalmic examination, including fluorescein angiography, OCT and ultrasound.
Results:
Fundoscopy revealed a small retinal tear in the left eye, for which she received laser treatment. In addition, choroidal masses were detected in both eyes. Her medical history of a pneumectomy for a bronchial carcinoid six years earlier together with recent elevated chromogranin A blood levels prompted a diagnosis of choroidal metastases. Subsequently, a Gallium-68 DOTANOC positron emitting tomography/computer tomography scan revealed a spinal cord metastasis and mediastinal as well as mesenterial lymph node invasion. Systemic treatment with Sandostatin®, a somatostatin analogue was started. Up until two years after the initial presentation and treatment, these choroidal lesions remained stable without any signs of growth.
Conclusion:
Endobronchial carcinoid tumors have an indolent nature and long-term follow-up is recommended for early detection of metastases. Although treatment with somatostatin analogues rarely induces complete tumor regression, tumor stabilization and prevention of symptoms related to hormone secretion is achieved. This well-tolerated systemic treatment provides a worthy alternative treatment for choroidal metastasis compared to classic radiotherapy without any risk of radiation or laser-related visual loss.
BACKGROUND
Carcinoid tumors constitute about 0.5% of all malignancies [1]. They originate most frequently from gastrointestinal (67%) and bronchopulmonary tissue (25%) [1]. They are characterized by their slow-growing nature and their ability to secrete several hormones [1, 2]. In the past, bronchopulmonary carcinoids were considered benign because of their generally indolent behavior [1]. At present, they are classified as malignant, since they can invade and metastasize to almost every organ, including the eye and ocular adnexa [3-7]. Metastatic spread to the choroid usually originates from bronchial carcinoid tumors [4-7]. Herein, we report a case of bilateral multifocal choroidal metastases from an endobronchial carcinoid treated with a somatostatin analogue.
RESULTS
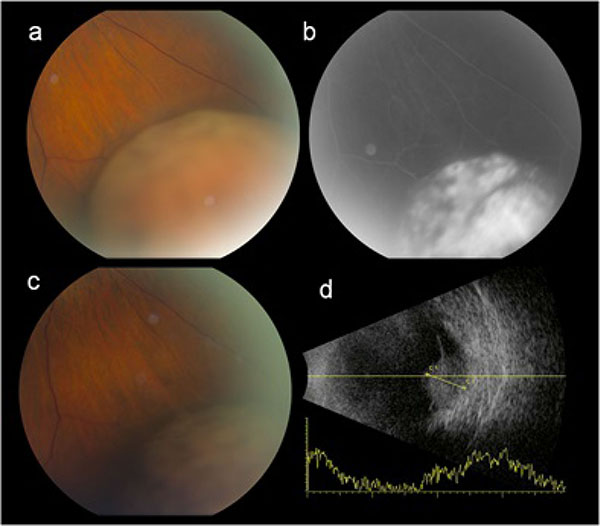
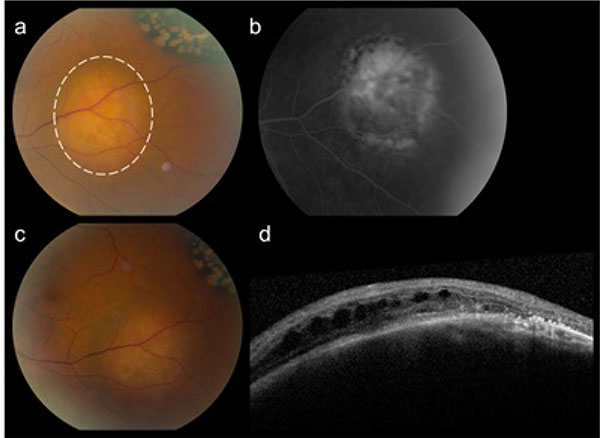
A 60-year-old woman presented with two weeks of photopsia in her left eye. No ocular history of high myopia or previous ocular trauma was recorded. Best-corrected visual acuity was 20/20 in both eyes. Slit-lamp examination was unremarkable. Fundus examination revealed a posterior vitreous detachment and a retinal tear with adjacent small retinal hemorrhage in the superior midperipheral retina of the left eye. The retinal tear was treated with laser photocoagulation therapy. Surprisingly however, there was an incidental finding of three distinct yellow choroidal masses: in the inferonasal periphery of the right eye (height 5.6 mm) (Fig. 1a, b) and in the inferotemporal macula (2.1 x 3.8 x 3.9 mm) (Fig. 2a, b) and inferonasal periphery of the left eye (height 3.6 mm) (Fig. 3a, b). Ultrasound examination of these lesions showed a moderate and uniform internal reflectivity (Fig. 1d) with a thickness between 2.1 and 5.6 mm. On the OCT scan there was intraretinal and subretinal fluid visible overlying the posterior pole lesion in the left eye (Fig. 2d).
The patient underwent a pneumectomy for an endobronchial carcinoid tumor six years earlier. Her daughter also had a carcinoid tumor. Molecular analysis for mutations in the MEN1 gene (multiple endocrine neoplasia type 1) to screen for an underlying genetic predisposition however was negative.
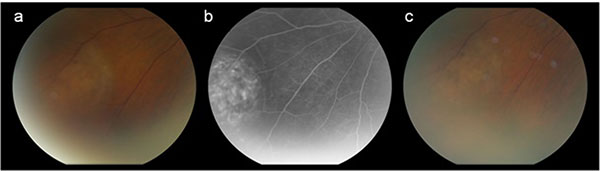 |
Fig. (3). The inferonasal choroidal carcinoid metastasis in the left eye. Color picture (a) and fluorescein angiography (b) at presentation. Same lesion after 10 cycli of Sandostatin® treatment (c). |
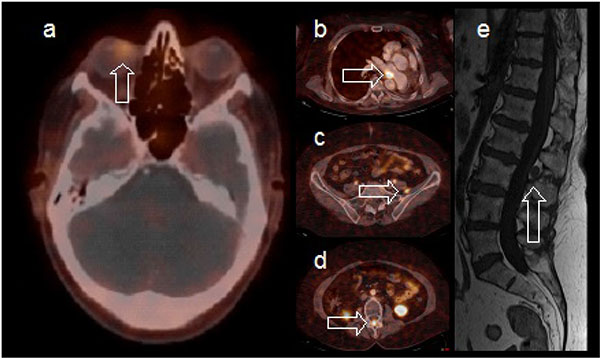
The ophthalmic examination and findings prompted a thorough systemic work-up with a PET/CT scan, a general blood test including chromogranin A level and a Gallium-68 DOTANOC PET/CT scan either to confirm the previous endobronchial carcinoid tumor as the source for the choroidal metastasis or to search for an unknown primary tumor. Laboratory results showed elevated chromogranin A levels (194 µg/L, reference values 40-170 µg/L), confirming carcinoid disease activity. A Gallium-68 DOTANOC PET/CT confirmed the presence of a large choroidal lesion in the right eye (Fig. 4a) and revealed an additional spinal cord metastasis on level L2-L3 (Fig. 4d, e) and mediastinal as well as mesenterial lymph node invasion (Fig. 4b, c). No novel non-carcinoid primary tumor was detected. This work-up together with her medical history, led to a clinical diagnosis of choroidal metastasis from the previous resected endobronchial carcinoid tumor.
A treatment with a somatostatin analogue (SSA), Sandostatin® LAR 30 mg, once a month, was started. The patient was followed for more than eighteen months without any signs of disease progression. One year after initiation of the Sandostatin® treatment, changes of the retinal pigment epithelium and outer retina were observed overlying the surface of the choroidal metastases illustrating their chronic nature (Figs. 1c, 2c and 3c). A stabilization in the volume of the choroidal metastases and no sign of growth nor decrease was noted. Reassessment with Gallium-68 DOTANOC PET/CT scan at six and eighteen months revealed no new metastatic lesions. The known systemic metastases were stable in comparison with the previous scan. Chromogranin A levels decreased from 194 µg/L to 137 µg/L after six months of treatment and were further reduced to 114 µg/L one year later.
DISCUSSION
Carcinoid activity can be monitored by serum analysis of chromogranin A, a protein stored in the neurosecretory vesicles of neuroendocrine tumor cells [2]. Chromogranin A is considered the most specific serum biomarker for carcinoid disease [8]. When the biochemical diagnosis of carcinoid disease is confirmed, a combination of imaging studies is required to localize the tumor [8]. As most carcinoid tumors contain high concentrations of somatostatin receptors, they can be imaged with the somatostatin analogue octreotide, labeled with the positron-emitting radioisotope Gallium-68 [9]. This technique is superior to 18F-labeled fluorodeoxyglucose positron emission tomography (FDG PET) in detecting neuroendocrine tumors [8].
After consultation with our colleagues of oncology, we have chosen to treat our patient suffering metastatic carcinoid disease with Sandostatin® LAR 30 mg. Synthetic analogues of native somatostatin, octreotide and lanreotide, are well-known medications to inhibit the secretion of biogenic amines by carcinoid tumors [10]. Although these analogues are highly effective in controlling symptoms of hormonal secretion, they are only rarely associated with complete tumor remission [2]. However, an increase in overall survival can be achieved by stabilization of the disease with subsequent prolongation of the progression-free survival (PFS) [10]. One year of follow-up during treatment with Sandostatin® LAR in our patient showed stabilization in the volume of the choroidal as well as the extraocular metastases. To our knowledge, this is the first report describing a stabilization of choroidal carcinoid metastases during treatment of systemic metastases with a SSA.
Further follow-up is recommended in order to detect any progression or complications early on. With risk of macular involvement of the lesion in the left eye and subsequent vision loss, photodynamic therapy with verteporfin could be taken into consideration, since this showed a significant decrease in tumor volume [11, 12]. Although external radiotherapy showed regression of choroidal carcinoid metastases [5], this treatment approach carries a considerable risk of visual loss due to radiation injury to the eye. Radiolabeled somatostatin analogues have also demonstrated a reduction in choroidal carcinoid metastases, but are limited in their use because of the risk of inducing hematologic toxicities and nephrotoxicity [13]. There is one case report describing long-term stabilization of choroidal carcinoid metastases without treatment at all [14]. Yet, the patient in the forementioned case report had only choroidal metastases and no other systemic involvement in contrast to our patient suffering advanced metastatic disease with multiple systemic metastases by which the stabilization of the spinal cord metastasis was of great concern. As such, we decided not to wait and to treat with a systemic SSA. This report is valuable in describing a patient with advanced metastatic carcinoid disease treated with a systemic SSA in which the choroidal metastases showed no signs of growth without local treatment.
CONCLUSION
Carcinoid tumors are rare and slow-growing neoplasms. Bronchial carcinoid tumors have a propensity to metastasize to the choroid. The diagnosis can be made by serum analysis of chromogranin A and imaging with a radiolabeled somatostatin analogue. Given the indolent nature of carcinoids, extensive and long-term follow-up of patients after resection of the primary tumor is recommended. This report complements previous literature and provides the useful new insight that choroidal metastases can be controlled with systemic somatostatin analogue treatment avoiding local treatment and potential radiation or laser-related visual loss.
LIST OF ABBREVIATIONS
| CT | = Computer Tomography |
| DOTANOC | = Ga-68- ,4,7,10-tetraazacyclododecane-N, ′,N′′,N′′′-tetraacetic acid-1-Nal3-octreotide |
| FDG PET | = 18F-labeled fluorodeoxyglucose positron emission tomography |
| MEN1 gene | = Multiple endocrine neoplasia type I gene |
| OCT | = Optical Coherence Tomography |
| PET | = Positron Emitting Tomography |
| Sandostatine LAR | = Sandostatine Long Acting Repeatable |
| SSA | = Somatostatin analogue |
INFORMED CONSENT
Informed consent was obtained from the patient.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.