RESEARCH ARTICLE
Ocular Tolerability of Preservative-Free Tafluprost and Latanoprost: in vitro and in vivo Comparative Study
Yoshihiko Esaki1, Atsushi Shimazaki1, Pertti Pellinen2, *
Article Information
Identifiers and Pagination:
Year: 2016Volume: 10
First Page: 146
Last Page: 153
Publisher ID: TOOPHTJ-10-146
DOI: 10.2174/1874364101610010146
Article History:
Received Date: 12/12/2015Revision Received Date: 12/05/2016
Acceptance Date: 12/05/2016
Electronic publication date: 31/05/2016
Collection year: 2016

open-access license: This is an open access article licensed under the terms of the (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, noncommercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Objective:
Detrimental effects of the preserved prostaglandin analogs (PGAs) have been thoroughly documented in the published literature. The current work studied two preservative-free (PF) prostaglandin eye drops: PF tafluprost and PF latanoprost. The aim of the study was to compare these two PF formulations in vitro for viability of the human corneal epithelial (HCE-T) cells and in vivo for ocular tolerability of the rabbit eye.
Method:
Viability of the HCE-T cells was measured by the MTS assay. The SV40-immortalized HCE-T cells were exposed to 100 µL of the drug solutions (at their commercial concentrations) or the culture medium. Ocular irritation was evaluated after repeated instillation of the drug solutions in Japanese white rabbits (Kbl:JW).
Results:
A significant loss of HCE-T cell viability was observed in vitro immediately after the exposure to PF latanoprost formulation but not immediately after the exposure to PF tafluprost formulation. Congruently, PF latanoprost induced in vivo more irritation on the rabbit eye than PF tafluprost.
Conclusion:
Comparing these two PF formulations in vitro and in vivo, it is considered that ocular tolerability of PF tafluprost is better than PF latanoprost. Taking into account the composition of these two PF PGA formulations, the solubilizing agent macrogolglycerol hydroxystearate 40 (MGHS40) contained in PF latanoprost formulation is a plausible cause for the negative effects.
INTRODUCTION
Glaucoma is the leading cause of irreversible visual impairment and blindness worldwide; it is a neuro-degenerative disease that involves the loss of retinal ganglion cells [1]. Elevated intraocular pressure (IOP) is the primary and only mutable risk factor for glaucoma [2]. Thus, topical IOP-lowering medications play a key role in preventing the progression of glaucoma [3]. As these medical therapies usually last for decades, they also need to be essentially safe and well tolerated. Today, prostaglandin analogs (PGAs) are used as the first-line treatment of glaucoma: latanoprost 0.05 mg/mL (Xalatan®, Pfizer, New York USA), travoprost 0.04 mg/mL (Travatan®, Alcon, Fort Worth, USA) and tafluprost 0.015 mg/mL (Taflotan® or Saflutan®, Santen Oy, Tampere, Finland) are all prodrugs of synthetic analogs of prostaglandin F2α (PGF2α) and lower IOP effectively by acting on the prostanoid FP receptors. Bimatoprost 0.1 mg/mL and 0.3 mg/mL (Lumigan®, Allergan, Irvine, USA), in turn, can be regarded as both a prostaglandin prodrug and a prostamide [4-6]. The pharmacological profiles of these compounds have been thoroughly presented and discussed in the published literature [7-10]. All four prostaglandin derivatives lower IOP by 25-35% and cause conjunctival hyperemia and eye irritation with different frequency. The highest rate of hyperemia has been reported for bimatoprost due to an inherent property of the compound [11, 12].
In addition to the direct pharmacological mechanism of action, the formulation of the PGA eye drops may also increase the risk of adverse ocular effects. The PGA molecules are lipophilic by nature and hence challenging to formulate as aqueous solutions. Therefore, excipients need to be added to the solutions that foster solubility and stability of the PGA. Furthermore, a preservative has to be included in all conventional multi-dose containers per se in order to secure sterility and stability of the product [13]. At present, the most commonly used preservative in ophthalmic formulations is benzalkonium chloride (BAC) – a quaternary ammonium salt. In antiglaucoma medications, BAC is typically used with concentrations ranging from 0.04 to 0.25 mg/mL. At these strengths, BAC is toxic in a dose-dependent manner and has deleterious effects on cornea, conjunctiva and trabecular meshwork [13-24]. It also causes and/or exacerbates pre-existing ocular surface disease (OSD) and contributes to the development cataracts in some patients [25-30]. Eventually, BAC may also predispose a patient to a greater risk of trabeculectomy failure [31], a surgical treatment option for the management of glaucoma.
To overcome the adverse effects of BAC, preservative-free (PF) ophthalmic formulations of the PGAs have been developed. Tafluprost 0.015 mg/mL was the first PF eye drop to enter the market [28, 32-34]. Today, also PF latanoprost 0.05 mg/mL (Monoprost®, Thea, Clermont-Ferrand, France) and PF bimatoprost 0.3 mg/mL are commercially available [35, 36]. The formulation of travoprost is still preserved with e.g. polyquaternium-1 (PQ-1), which is known to evoke cytotoxicity and enhance NF-κB-driven inflammation in human corneal epithelial (HCE-2) cells [37-43]. In parallel, however, the excipients targeted to facilitate the solubility and stability of the PF PGA may also induce detrimental effects in the eye. The results of a recent publication affirmed indeed that the solubilizing agent macrogolglycerol hydroxystearate 40 (MGHS40) contained in PF latanoprost formulation was associated with IL-6-mediated inflammatory response and increased cytotoxicity in the HCE-2 cell cultures [44]. No such findings were observed with the formulation of PF tafluprost. The motivation of this paper was to delve further into these two unit-dose PF PGA formulations (i) by evaluating in vitro the effects of PF tafluprost and PF latanoprost on the viability of human corneal epithelial (HCE-T) cells and (ii) by comparing in vivo the ocular tolerability of these PF PGAs [45].
MATERIALS AND METHODOLOGY
Cell Viability Assay
SV40-immortalized HCE-T cells (RIKEN BRC, Japan) were exposed inter alia to 100 µl of the drug solutions (PF tafluprost or PF latanoprost at their commercial concentrations) or the culture medium for the epithelial cells – i.e. DMEM/F-12 (Nacalai Tesque, Japan) supplemented with 10% FFS (Bio West, France) - in a 96-well plate and kept in the CO2 incubator (Panasonic, Japan) at 5% CO2 and 37° C for 5, 15, 30, or 60 minutes.
Viability of the cells was determined by the MTS assay: for each time point, absorbance at the wavelength of 490 nm was measured using the 3550 Microplate Reader BIO-RAD, USA). The final absorbance value was obtained after subtracting the average of blank cells from the measured value. Relative absorbance was calculated as the ratios of drug solutions to the medium (which equals to 100%) at each time point of measurement.
Ocular Irritation Study
Japanese male white rabbits (Kbl:JW) weighing 1.4-2.0 kg were instilled 50 µl of the drug solutions (PF tafluprost or PF latanoprost at their commercial concentrations) on the left eye 10 times a day at 30 minutes intervals; the contralateral eye remained as an untreated control. The study followed a two-period cross-over design: at the initial dosing, the first half of the rabbits (nos. 1-3) received PF latanoprost and the second half (nos. 4-6) PF tafluprost. At the second dosing, after a 6 days washout period, the rabbits switched from PF latanoprost to PF tafluprost and vice versa.
Ocular irritation of the treated eye was determined macroscopically by the McDonald-Shadduck scoring system [46]: conjunctival hyperemia, swelling and discharge were evaluated at 30 minutes, 3 and 5 hours, and 1 day after the last instillation. Cornea, anterior chamber and iris were investigated at the same points of time using a slit lamp. Fluorescein staining of the cornea was evaluated macroscopically at 5 hours after the last instillation and once during the following day. The observer was masked with regard to the identity of the drug solutions.
All the procedures followed were in accordance with the standards set forth in the eight edition of Guide for the Care and Use of Laboratory Animals by the National Academy of Sciences.
Statistical Methods
All results were presented as mean ± standard deviation (SD) unless otherwise indicated. Analysis of variance (ANOVA) model was fitted at each time point for absorbance, and Dunnett’s correction was applied for the pairwise comparisons of drug solutions (PF latanoprost and PF tafluprost) versus the medium. Similarly, two-sample t-test (one-sample t-test) was used to compare the relative absorbance (irritation scores) between the drug solutions. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Cell Viability
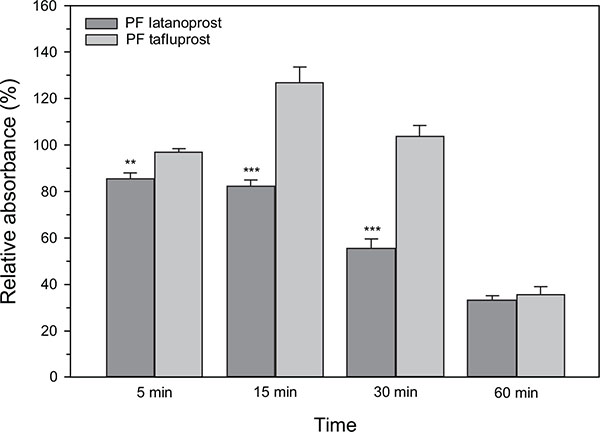
The effects of PF tafluprost and PF latanoprost on HCE-T cells - as measured by the MTS assay - are presented in Fig. (1). Overall, cell viability of the culture medium remained stable over the 60 minutes investigational period; the mean absorbance (of four wells) ranged merely from 0.306 ± 0.031 at 5 minutes to 0.367 ± 0.045 at 30 minutes. The wells that were exposed to PF latanoprost, in turn, had the lowest absorbance of 0.262 ± 0.016 already at 5 minutes. After 15 minutes, the absorbance with PF latanoprost started to decline rapidly in a time-dependent manner; that is, from 0.295 ± 0.019 at 15 minutes to 0.110 ± 0.037 at 60 minutes. Hence, statistically significant loss of HCE-T cell viability versus the culture medium was seen at each time point for PF latanoprost. Conversely, such decreases were not seen for PF tafluprost during the first half an hour; the absorbance was 0.297 ± 0.009 at 5 minutes and 0.382 ± 0.030 at 30 minutes. At 60 minutes the viability had diminished in a comparable manner with both PF formulations. Subsequently, the largest differences in relative absorbance between the drug solutions were seen at 15 and 30 minutes (Fig. 1; p < 0.001 for PF tafluprost vs. PF latanoprost at both time points).
Ocular Irritation
Individual and average results of the ocular irritation variables are presented in Table 1 and Fig. (2). Conjunctival hyperemia, swelling and discharge in rabbit eye were all observed with both drug solutions and they appeared to peak at 30 minutes after the last instillation. Each of these three adverse ocular effects was, however, more prominent and severe with PF latanoprost than with PF tafluprost. At 30 minutes, the mean scores of conjunctival hyperemia were still alike (1.67 ± 0.52) for PF tafluprost and PF latanoprost. By 5 hours, conjunctival hyperemia was essentially recovered with PF tafluprost opposite to PF latanoprost for which mild/moderate hyperemia was still widely seen. The mean scores at 5 hours were 0.33 ± 0.52 and 0.83 ± 0.75 (Fig. 2; p < 0.05). At 30 minutes, the mean scores of conjunctival swelling were 0.33 ± 0.52 for both PF treatments. At 3 hours, no swelling was seen with PF tafluprost and one mild case was detected with PF latanoprost. At 30 minutes, the mean scores of conjunctival discharge were 0.50 ± 0.84 for PF tafluprost and 0.67 ± 1.03 for PF latanoprost. One case of mild discharge was still present at 3 hours for both PF treatments. In general, no abnormal findings were seen at the cornea, anterior chamber and iris for either drug formulation with slit lamp examination and fluorescein staining of the cornea.
The results of ophthalmological examinations.
| Drug formulation | PF latanoprost (Monoprost®) | PF tafluprost (Taflotan®) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irritation variable1 | Time point2 | 1 | 2 | 3 | 4 | 5 | 6 | mean±SD | 1 | 2 | 3 | 4 | 5 | 6 | mean±SD |
| Conjunctival hyperemia | 30 minutes | 2 | 2 | 2 | 1 | 1 | 2 | 1.67±0.52 | 1 | 2 | 2 | 2 | 2 | 1 | 1.67±0.52 |
| 3 hours | 2 | 2 | 1 | 0 | 1 | 2 | 1.33±0.82 | 1 | 1 | 1 | 1 | 2 | 1 | 1.17±0.41 | |
| 5 hours | 1 | 2 | 1 | 0 | 0 | 1 | 0.83±0.754 | 0 | 0 | 0 | 0 | 1 | 1 | 0.33±0.52 | |
| 1 day | 0 | 1 | 0 | 0 | 0 | 0 | 0.17±0.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00±0.00 | |
| Conjunctival swelling3 | 30 minutes | 1 | 1 | 0 | 0 | 0 | 0 | 0.33±0.52 | 0 | 0 | 1 | 0 | 1 | 0 | 0.33±0.52 |
| 3 hours | 1 | 0 | 0 | 0 | 0 | 0 | 0.17±0.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00±0.00 | |
| Conjunctival discharge3 | 30 minutes | 2 | 2 | 0 | 0 | 0 | 0 | 0.67±1.03 | 0 | 0 | 0 | 1 | 2 | 0 | 0.50±0.84 |
| 3 hours | 1 | 0 | 0 | 0 | 0 | 0 | 0.17±0.41 | 0 | 0 | 0 | 0 | 1 | 0 | 0.17±0.41 | |
1 Each number represents an individual score of ocular irritation by the McDonald-Shadduck scheme: 0=Normal, 1=Slight and 2=Moderate
2 Time after final instillation; left eyes were treated and right eyes were untreated; all scores were zeros for the untreated eyes
3 All scores were zeros at 5 hours and 1 day
4 p<0.05 still with PF latanoprost vs. zero mean (unlike PF tafluprost)
DISCUSSION
The present study compared two distinct PGAs that are available as commercial PF formulations in sterile unit-dose containers; namely, PF tafluprost and PF latanoprost. Other PGAs were not included in the evaluation, since they either had slightly disparate mechanism of action leading by default to a higher risk of ocular irritation (bimatoprost) or were solely available as a preserved formulation in a multi-dose container (travoprost). Specifically, the removal of the preservative from the formulation is of utmost essence, as the most frequently used preservative agents BAC and PQ-1 (a detergent-type derivative from BAC used with travoprost in Europe) have already proven to evoke cytotoxic effects in vitro and induce inflammation on the ocular surface cells [13-24, 37-43]. Therefore, for example, the clinical use of BAC (at 0.04 to 0.25 mg/mL) correlates well with the signs and symptoms of OSD and the failure rate of glaucoma filtration surgery [25-31].
Evidently, the aforementioned harmful effects do not materialize at low strengths of BAC [47]: tafluprost preserved with 0.01 mg/mL of BAC was shown not to be cytotoxic in contrast to travoprost preserved with Sofzia® (an ionic-buffered preservative used in the US) and bimatoprost, travoprost, and latanoprost preserved with 0.05 mg/mL 0.15 mg/mL, and 0.2 mg/mL of BAC, respectively [16, 41]. Sometimes, it has been alleged that these preservatives facilitate the penetration of the PGA into the eye through their detergent activity and hence intensify the efficacy of the drug [48]. However, many studies have now made this past belief close to obsolete and shown that switch to a PF formulation shall, in fact, enhance treatment adherence and efficacy as a result of improved safety and tolerability [28, 32-36]. The PF PGAs have thus directly influenced the clinical guidelines as well as the cost-benefit assessment of the glaucoma treatments [49, 50].
Entirely divergent approaches were undertaken to ensure the solubility of the PGA in PF tafluprost and PF latanoprost formulations: non-ionic surfactants P80 (0.75 mg/mL in PF tafluprost) and MGHS40 (50 mg/mL in PF latanoprost) were utilized as solubilizers, accordingly. The concentration of MGHS40 in PF latanoprost is exceptionally high; it is precisely 1000-fold when compared to that of latanoprost and 250-fold when compared to that of BAC (0.2 mg/mL) in the preserved form of latanoprost. Until recently, information concerning the ocular safety of MGHS40 has been far too scarce - bearing in mind the fact that high concentrations of surface-active compounds are known compromise the integrity of cell membranes [51]. In a seminal paper, finally, the HCE-2 cells were exposed to diluted PF tafluprost, PF latanoprost, BAC, and MGHS40 for 1, 6, 12, 24 and 48 hours, and to undiluted once daily PF tafluprost and PF latanoprost for 9 days [44]. The results of this in vitro study inarguably demonstrated that diluted PF latanoprost, BAC and MGHS40 all exerted concentration and time dependent cellular damage and inflammation, while no relevant morphological changes were displayed by PF tafluprost. Undiluted PF latanoprost also increased significantly the release of LDH and secretion of IL-6 in contrast to undiluted PF tafluprost.
In the present study both in vitro and in vivo methods were used. The cell viability assay clearly demonstrated a difference between the two groups up to 30 minutes of incubation. After one hour the viability decreased significantly in both groups probably reflecting the general study conditions and the sensitivity of the method used. When the hyperemia was assessed no difference between groups could be observed after 30 minutes and 3 hours. This can be explained by the ability of prostaglandin analogs to cause vasodilatation which could initially mask the hyperemia caused by ocular irritation. After 5 hours there was a statistically significant difference between groups indicating irritation induced slower recovery rate of PF latanoprost treated animals.
In essence, the findings of this study provided supplemental in vitro evidence on the detrimental effects of PF latanoprost on the viability of HCE-T cells. Alike, it was further demonstrated that these effects can be seen in vivo when the product is administered topically onto the rabbit eye. On the basis of the earlier results [44] and the fact that other components of the two formulations are well known and widely used in eye drops, the emerged differences between the two PGAs - PF tafluprost and PF latanoprost - can plausibly be attributed to the high concentration of excipient MGHS40.
CONCLUSION
Larger complementary studies are definitively needed to characterize the negative effects of PF latanoprost (and MGHS40) in greater detail. Above all, however, much more attention must be paid to the investigation of the additional excipients included in the ophthalmic formulations – some of them seem to cause similar deleterious effects in vitro and in vivo as the preservatives.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Jouni Vuorinen from Oy 4Pharma Ltd for valuable contribution to the preparation of the manuscript.