RESEARCH ARTICLE
Efficacy and Safety of Switching from Prostaglandin Analog Therapy to Prostaglandin / Timolol Fixed Combination or Prostaglandin / Brimonidine Therapy
Kenji Inoue1, *, Mieko Masumoto1, Kyoko Ishida2, Goji Tomita2
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
First Page: 156
Last Page: 163
Publisher ID: TOOPHTJ-11-156
DOI: 10.2174/1874364101711010156
Article History:
Received Date: 28/01/2017Revision Received Date: 16/02/2017
Acceptance Date: 04/04/2017
Electronic publication date: 30/06/2017
Collection year: 2017

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
To compare the safety and efficacy between switching to prostaglandin/timolol fixed combination eye drops (PG/timolol FCs) and adding brimonidine to PG analogue monotherapy.
Methods:
Eyes of 53 patients with primary open-angle glaucoma or ocular hypertension who were receiving PG analogue monotherapy were included. Participants were randomly divided into two treatment groups: one was prescribed PG/timolol FCs (switched group), and for the other, 0.1% brimonidine was added to the PG analogue (added group). Intraocular pressure (IOP), blood pressure, and pulse rate were measured after 1 and 3 months and compared to baseline values. Participants were also surveyed to determine if they had experienced systemic or topical adverse events at each study visit. IOP changes at 1 and 3 months were compared between groups.
Results:
Three months after changing medication, mean IOP was 14.6 ± 2.4 mmHg in the switched group and 13.7 ± 1.8 mmHg in the added group; both were significantly lower than the baseline values (switched group, 16.5 ± 2.7 mmHg; added group, 15.8 ± 2.3 mmHg; both P < 0.001). Neither the mean nor the percentage reductions in IOP were significantly different between groups at 1 and 3 months. In the added group, diastolic blood pressure was lower than that at 1 and 3 months, systolic blood was lower than that at 3 months (P < 0.01). The patients who had experienced systemic or topical adverse events were 53.8% in the added group and 40.7% in the the changed group, which was equivalent between groups (P =0.4142). Three patients (11.5%) in the added group, but none from the switched group, were excluded from analyses because of adverse events (not significant, P = 0.217).
Conclusion:
Switching from a PG analogue to PG/timolol FCs or to PG with brimonidine was equally safe (systemically and topically) and effective in reducing IOP. Thus, PG with brimonidine might be appropriate medication in patients who cannot use PG/timolol FCs due to repiratory or circulatory disease.
INTRODUCTION
Glaucoma is often initially treated with a single medication that lowers intraocular pressure (IOP). Prostaglandin (PG) analogues are generally the first choice for glaucoma treatment because they are effective in reducing IOP, have few systemic adverse effects, and offer a convenient once-daily administration protocol. However, when PG analogues fail to sufficiently reduce IOP, a medication change or addition is needed. Switching patients from PG analogue monotherapy to PG/timolol fixed combination eye drops (PG/timolol FCs) is common [1] because of high patient compliance and the continued once-daily administration protocol. However, PG/timolol FCs, which contain a β-blocker, might not be used in patients with respiratory or circulatory disease. Fortunately, topical carbonic anhydrase inhibitors and α-2 stimulators might be both appropriate medication additions for patients who cannot use β-blockers. Brimonidine, an α2-adrenergic agonist, has been shown to have few systemic adverse effects, is effective in reducing IOP, and slows down the progression of visual field defects (i.e., neuroprotective activity) [2]. Therefore, switching patients to PG/timolol FCs is favorable for patient compliance, while by adding brimonidine to PG analogue therapy, except greater IOP reduction, we might expect to achieve additional IOP-independent neuroprotection. In Japan, brimonidine 0.1% has only been approved for use as an adjunctive therapy since 2012.
A direct comparison between patients treated with PG analogue monotherapy who had switched to PG/timolol FCs with those who had brimonidine added to their medication regimen has not yet been performed in Japan. Here, we prospectively investigate the systemic and topical safety and efficacy of reducing IOP in Japanese patients by switching to PG/timolol FCs or by adding brimonidine to their glaucoma treatment regimen.
MATERIALS AND METHODS
This study was conducted between April 2014 and October 2015 at the Inouye Eye Hospital (Tokyo, Japan). The protocol was approved by the hospital’s ethics committee and all participants provided written informed consent before any study procedure or examination was performed. All study conduct adhered to the tenets of the Declaration of Helsinki.
Study subjects had primary open-angle glaucoma (POAG) or ocular hypertension (OH) that was being treated with PG analogue which contains preservatives monotherapy administered once daily in the evening. All subjects showed insufficient IOP reduction after more than 3 months of latanoprost or travoprost treatment, necessitating a change in IOP-lowering medication. Subjects whose IOP reduction rate was < 20% using PG analogue monotherapy or visual field defects had increased were defined as IOP insufficient cases. Subjects with corneal disease whose IOP could not be precisely measured or who had undergone cataract surgery in the past 3 months were excluded from participation. In cases where both eyes qualified for study inclusion, the eye with the higher IOP was selected as the study eye. If both eyes had the same IOP, the right eye was selected as the study eye.
Participants were instructed to discontinue PG analogue in the study eye and to begin using study medication instead. Medications were not changed in the non-study eye. Subjects were randomly divided into two treatment groups using sealed envelopes. One treatment group began using PG/timolol FCs which contains preservatives monotherapy once daily in the evening (switched group) and one treatment group began using both a PG analogue (once daily in the evening) and 0.1% brimonidine which contains preservatives monotherapy (twice daily in the morning and evening; added group). In the switched group, 0.005% latanoprost was replaced by 0.005% latanoprost/0.5% timolol FCs and 0.004% travoprost was replaced by 0.004% travoprost/0.5% timolol FCs.
All subjects underwent ophthalmic examination, including measurement of IOP (Goldmann tonometry) before (baseline), 1 and 3 months after the use of the study medication and 30-2 SITA Standard Humphrey visual field testing before administration. Blood pressure (pulsimeter, UDEX super TYPE, Elquest Co., Ltd., Chiba, Japan) and pulse rate (pulsimeter, UDEX super TYPE, Elquest Co., Ltd., Chiba, Japan) were also measured at each time point. Topical adverse reactions were examined with slit-lamp microscopy and consultation. The IOP, blood pressure, and pulse rate were compared between baseline and 1 and 3 months within groups using paired t tests, and between groups at each time point using unpaired t tests. The reduction in IOP from baseline to 1 and 3 months, and the rate of reduction, were calculated and analyzed using the Wilcoxon signed rank test. Participants who dropped out of the study before the end of the 3-month observation period were also investigated. A P value < 0.05 was considered statistically significant.
RESULTS
A total of 53 eyes of the 53 patients (21 men, 32 women) were included in this study. Mean subject age at baseline was 64.5 ± 12.4 years (range, 38–85 years). A total of 52 eyes had POAG, including 33 eyes with normal-tension glaucoma, and one eye had OH. Before beginning the study medication, 40 patients had been using latanoprost and 13 patients had been using travoprost for a period of 89 ± 42 months (range, 18-186 months).
Twenty-seven patients were assigned to the switched group and 26 patients were assigned to the added group Table (1). At baseline, there were no significant differences between the groups in gender, glaucoma type, pretreatment medications, number of medications used before the study, PG analogue administration period, baseline IOP, visual field mean deviation (MD), systolic blood pressure, diastolic blood pressure, and pulse rate. However, the subject age was significantly higher in the added group than in the switched group (p = 0.0034).
| Switched Group | Added Group | P Value | |
|---|---|---|---|
| n (patients) | 27 | 26 | --- |
| Male: Female | 13:14 | 8:18 | 0.264 |
| Age (years) | 59.7 ± 12.0 (42–85) | 69.4 ± 10.9 (38–83) | 0.0034 |
| Systemic Diseases | 4 | 7 | 0.3265 |
| Glaucoma Type | 0.217 | ||
| POAG | 12 | 7 | |
| NTG | 14 | 19 | |
| OH | 1 | 0 | |
| Pretreatment medications | 0.526 | ||
| Latanoprost | 19 | 21 | |
| Travoprost | 8 | 5 | |
| PG treatment period (months) | 88 ± 43 (32–168) | 89 ± 41 (18–186) | 0.838 |
| IOP (mmHg) | 16.5 ± 2.7 (11–24) | 15.8 ± 2.3 (11–20) | 0.278 |
| Visual field MD (dB) | -9.69 ± 5.70 (-22.81–0.65) | -8.71 ± 5.84 (-17.99–1.18) | 0.610 |
| Systolic BP (mmHg) | 129 ± 21(94–175) | 135 ± 16(110–172) | 0.314 |
| Diastolic BP (mmHg) | 77 ± 12(50–98) | 76 ± 8(58–94) | 0.676 |
| Pulse rate | 74 ± 10(56–97) | 73 ± 9(51–86) | 0.778 |
POAG, primary open-angle glaucoma; NTG, normal-tension glaucoma; OH, ocular hypertension; PG, prostaglandin; IOP, intraocular pressure; MD, mean deviation; BP, blood pressure
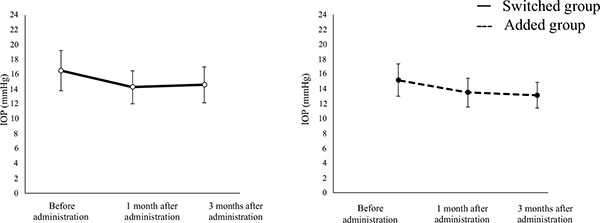
The mean IOP was significantly lower in the switched group after 1 (14.3 ± 2.2 mmHg) and 3 (14.6 ± 2.4 mmHg) months of study medication use compared with that at baseline (16.5 ± 2.7 mmHg, both p < 0.0001). Similar results were obtained in the added group (baseline IOP, 15.8 ± 2.3 mmHg; 1-month IOP, 14.1 ± 2.0 mmHg; 3-month IOP, 13.7 ± 1.8 mmHg; both P < 0.001; (Fig. 1). The mean reduction in IOP after 1 month was 2.2 ± 2.1 mmHg in the switched group and 1.7 ± 2.5 mmHg in the added group; after 3 months, it was 2.0 ± 2.3 mmHg in the switched group and 2.1 ± 2.3 mmHg in the added group. These slight differences between the two groups were not statistically significant (1 month, P = 0.203; 3 months, P = 0.907). The IOP reduction rate at 1 month was 12.7 ± 11.3% in the switched group and 9.3 ± 14.1% in the added group (P = 0.185). The IOP reduction rate at 3 months was 11.2 ± 12.2% in the switched group and 12.1 ± 11.8% in the added group (P = 0.922). Again, these slight differences between groups were not statistically significant.
| Baseline | 1 month | 3 months | |
|---|---|---|---|
| Systolic BP (mm Hg) | |||
| Switched group | 129 ± 21 | 127 ± 18 | 124 ± 18 |
| Added group | 135 ± 16 | 128 ± 19 | 124 ± 21* |
| Diastolic BP (mmHg) | |||
| Switched group | 77 ± 12 | 74 ± 11 | 76 ± 12 |
| Added group | 76 ± 8 | 68 ± 8** | 71 ± 10* |
| Pulse rate (beats/minute) | |||
| Switched group | 74 ± 10 | 74 ± 10 | 73 ± 9 |
| Added group | 73 ± 9 | 73 ± 9 | 75 ± 11 |
BP, blood pressure
Neither systolic nor diastolic blood pressure measurements differed from baseline in the switched group after 1 and 3 months of study medication use Table (2). In the added group, however, systolic blood pressure at 3 months (p < 0.01) and diastolic blood pressure at 1 (P < 0.001) and 3 (P < 0.01) months were significantly lower than baseline. Pulse rate did not change from baseline at 1 or 3 months in either study group (1 month, P = 0.940; 3 months, P = 0.572). In the switched group, four patients (14.8%) developed systemic diseases, including hypertension (2 patients), hyperlipidemia (1 patient), and high blood pressure + hyperlipidemia (1 patient). In the added group, seven patients (26.9%) developed systemic diseases, including hypertension (2 patients), gout (1 patient), rheumatism (1 patient), prostatic hyperplasia (1 patient), diabetes (1 patient) and gastritis (1 patient) (Fisher's exact test, P = 0.3265).
| Onset date | Adverse events | Medication status following adverse event(s) | ||
|---|---|---|---|---|
| Switched group | 1 month | 1 case | Ocular stimulation | Continuation |
| 1 month | 1 case | Eyelid pigmentation | Continuation | |
| 1 month | 1 case | Superficial punctate keratopathy | Continuation | |
| 1 month | 1 case | Impaired vision | Continuation | |
| 3 months | 2 cases | Itchiness | Continuation | |
| 3 months | 4 cases | Impaired vision | Continuation | |
| 3 months | 1 case | Eye-smarting | Continuation | |
| Added group | 1 month | 1 case | Palpitation, Chest pain | Discontinuation |
| 1 month | 1 case | Bradycardia, Somnolentia | Discontinuation | |
| 1 month | 1 case | Oppressive feeling | Continuation | |
| 1 month | 1 case | Eye strain | Continuation | |
| 1 month | 1 case | Dizziness | Continuation | |
| 1 month | 1 case | Discomfort | Continuation | |
| 1 month | 3 cases | Impaired vision | Continuation | |
| 1 month | 1 case | Hyperemia | Continuation | |
| 3 months | 1 case | Ocular irritation | Discontinuation | |
| 3 months | 1 case | Discomfort | Continuation | |
| 3 months | 1 case | Blepharospasm | Continuation | |
| 3 months | 1 case | Itchiness | Continuation | |
Adverse events were experienced by 11 patients (40.7%) in the switched group, and 14 patients (53.8%) in the added group (Fisher’s exact test, P = 0.4112) Table (3). Three of the added group patients (11.5%), but none of the switched group patients, were excluded from analyses due to these systemic or topical adverse events. This slight difference in the number of excluded patients between groups was not significant (P = 0.217). In the added group, one subject experienced palpitations and chest pain, one had bradycardia and somnolentia, and one experienced ocular irritation.
DISCUSSION
One study reported that adding brimonidine to PG analogues monotherapy or switching to a PG/β-blocker fixed combination are common practices that are favorable for patients [1]. However, the safety and efficacy of adding brimonidine to PG analogues monotherapy or switching to PG/β-blocker fixed combinations have not been investigated. Here, we examined the safety and efficacy in this study. Several studies have previously reported efficacy in IOP reduction when switching from PG analogues to PG/timolol FCs Table (4) [3-11]. When switching from latanoprost to latanoprost/timolol fixed combination eye drops, the IOP was reduced by 2.1-4.71 mmHg, or 11.2-19.5%, during the follow-up period from 2 to 6 months [3-7]. When switching from travoprost to travoprost/timolol fixed combination, IOP was reduced by 2.6-3.6 mmHg, or 13.6-28.5%, during the follow-up period from 3 to 24 months [8-11]. These IOP reductions are equivalent or lower than those previously reported [3-11]. However, baseline IOP (16.5 ± 2.7 mmHg) in our study subjects was lower (> 20 mmHg) than that of previous studies [3-11], which could explain this difference. The IOP reduction achieved by adding brimonidine to PG analogue therapy has also been examined previously Table (5) [12-20]. Brimonidine 0.1% has been used in Japan since 2012, but brimonidine 0.15% and 0.2% are used worldwide. In two reports of brimonidine 0.2% added to PG analogues, IOP was reduced by 2.3-5.9 mmHg and 13.4-32.2% during 1- to 2-month follow-up periods [12, 13]. In others, brimonidine 0.15% added to PG analogues delivered a 2.0–5.1 mmHg / 9–23% IOP reduction (6-week to 3-month follow-up) [14-16], while IOP reductions from a 0.1% brimonidine addition were 1.5-3.3 mmHg / 11.8-16.8% (4-week to 12-month follow-up) [17-22]. In agreement with prior studies [12-20], we found that mean IOP was 14.6 ± 2.4 mmHg over a 3-month follow-up period. In the current study, mean IOP was reduced by 2.0 ± 2.3 mmHg or 11.2 ± 12.2% over a 3-month (12-week) follow-up period.
| n (patients) | Prostaglandin/timolol FCs | Pre-treatment IOP (mmHg) | IOP reduction (mmHg) | IOP reduction rate (%) | |
|---|---|---|---|---|---|
| Latanoprost→Latanoprost/timolol fixed combination | |||||
| Hamacher T. Br J Ophthalmol. 2004 [3] | 69 | 2 months | 20.8 ± 3.4 | 3.1 ± 3.6 | 14.9 |
| Dunker S. Adv Ther. 2007 [4] | 51 | 6 months | 20.7 ± 3.6 | 2.9 ± 2.8 | 14.0 |
| Polo V. Ann Ophthalmol. 2008 [5] | 33 | 3 months | 20.38 ± 5.33 | 4.71 | 19.5 |
| Kitazawa Y. Rinsho Ganka. 2009 [6] | 144 | 8 weeks | 19.62 ± 2.60 | 2.59 ± 2.40 | 13.2 |
| Inoue K. Clin Ophthalmol. 2012 [7] | 31 | 6 months | 17.3 ± 2.7 | 2.1 ± 2.3 | 11.2 ± 11.8 |
| Travoprost→Travoprost/timolol fixed combination | |||||
| Mandic Z. Methods Find Exp Clin Pharmacol. 2010 [8] | 45 | 3 months | 22 | 4.4 ± 2.8 | 20 |
| Pfeiffer N. Clin Ophthalmol. 2010 [9] | 45 | 12 weeks | 22.1 ± 2.7 | 6.3 ± 2.5 | 28.5 |
| Costa VP. Clin Ophthalmol. 2012 [10] | 43 | 12 weeks | 20.5 ± 2.1 | 3.9 | 19 |
| Muraki T. Rinsho Ganka. 2015 [11] | 34 | 2 years | 16.9 ± 3.3 | 2.6 ± 2.9 | 13.6 ± 14.9 |
| This study: PG analogue → PG/timolol fixed combination | |||||
| 27 | 12 weeks | 16.5 ± 2.7 | 2.0 ± 2.3 | 11.2 ± 12.2 | |
IOP, intraocular pressure; PG, prostaglandin
Previous studies have also examined the usefulness of a change in medication to improve IOP management. One study examined the effect on IOP when patients with POAG and OH who had been using PG analogue and timolol concomitantly switched from timolol to 0.1% brimonidine [21]. In that study, IOP of 12 weeks after switching medications (14.0 ± 2.8 mmHg) was significantly lower than baseline (15.7 ± 2.7 mmHg).
Reis et al. [13] compared the efficacy between adding 0.2% brimonidine or timolol to PG analogue therapy. When brimonidine was added, the reductions and percentage change in mean IOP were 2.3 ± 1.8mmHg and 13.4 ± 9.1%, respectively. When timolol was added, reductions were even greater at 3.9 ± 1.8 mmHg and 20.2 ± 7.5%, respectively, both of which were significantly larger than for brimonidine [13].
Several comparative trials have examined the efficacy of brimonidine and timolol in reducing IOP [2, 22-25]. Krupin et al. [2] reported an equivalent IOP reduction efficacy between brimonidine and timolol, but Araie et al. [22] and Konstas et al. [23] reported that timolol use resulted in a greater IOP reduction. The peak IOP was reportedly the same in eyes treated with brimonidine or timolol; however, the trough IOP was lower in eyes treated with timolol than in those treated with brimonidine [24, 25]. In the current study, mean reductions and percentage changes in IOP were the same when PG analogue monotherapy was switched to either PG/timolol FCs or PG analogue with brimonidine therapy. When using a single PG analogue, the IOP reduction efficacy of timolol is greater than that of brimonidine [22, 23]. However, when additional eye drops were used with PG analogues, IOP reduction efficacy was equivalent between PG/timolol FCs and brimonidine added to PG analogues.
This study also examined the effect of study medications on blood pressure and pulse rate. Both brimonidine and timolol are known to mechanically lower blood pressure and pulse rate, and we found that brimonidine did lower these parameters. In the added group, similarly to previous studies [22], diastolic blood pressure was lower after brimonidine administration than before administration. However, the reduction was small and blood pressure remained within normal limits. Therefore, although the change was statistically significant, it was not clinically relevant.
| n (patients) | Drug | Treatment period | IOP before brimonidine (mmHg) | IOP reduction width (mmHg) | IOP reduction rate (%) | |
|---|---|---|---|---|---|---|
| Brimonidine 0.2% | ||||||
| Lee DA. J Glaucoma. 2001 [12] | 16 | Latanoprost | 2 months | 18.3 | 5.89 | 32.2 |
| Reis R. Clin Ther. 2006 [13] | 16 | Travoprost | 4 weeks | 17.0 ±3.1 | 2.3 ± 1.8 | 13.4 ± 9.1 |
| Brimonidine 0.15% | ||||||
| Konstas AGP. Ophthalmology. 2005 [14] | 29 | Latanoprost | 6 weeks | 19.0 ± 1.7 | 2.2 ± 1.5 | 11.6 |
| Mundorf T. Adv Ther. 2007 [15] | 43 | Latanoprost | 2 months | 21.9 | Peak 5.1 Trough 2.0 |
23 9 |
| Feldman RM. Ophthalmology. 2007 [16] | 79 | Travoprost | 3 months | 21.7 ± 0.33 | 2.1 ± 0.27 | 9.7 |
| Brimonidine 0.1% | ||||||
| Day DG. Curr Med Res Opin. 2008 [17] | 20 | Latanoprost | 3 months | 19.6 ± 2.94 | 3.3 ± 2.82 | 16.8 |
| Araie M. Atarasii Ganka. 2012 [18] | 59 | PG analogues | 52 weeks | 18.7 | 2.7 | 14.4 |
| Yamamoto C. Atarasii Ganka. 2014 [19] | 24 | PG analogues | 3 months | 18.0 ± 2.7 | 2.1 ± 2.2 | 11.8 ± 11.4 |
| Hayashi Y. Rinsho Ganka. 2015 [20] | 17 | PG analogues | 12 months | 11.5 ± 1.5 | 1.5 ± 1.3 | 13.3 ± 10.9 |
| This study (latanoprost or travoprost) | ||||||
| 26 | PG analogues | 12 weeks | 15.8 ± 2.3 | 2.1 ± 2.3 | 12.1 ± 11.8 | |
IOP, intraocular pressure; PG, prostaglandin.
Prior studies had a dropout rate of 1.5–6.5% because of adverse events when subjects switched from PG analogue monotherapy to PG/timolol FCs [6, 7, 10, 11]. Adverse effects included conjunctive hyperemia, keratitis, ocular irritation and itchiness, bradycardia, and blurred vision. However, none of our switched group subjects withdrew from the study because of adverse reactions. In other studies, up to 23.7% of patients dropped out because of adverse reactions after adding brimonidine to PG analogue therapy [13, 14, 16-20]. Adverse effects included increased blood pressure, reduced blood pressure, bradycardia, headache, tinnitus, and ocular itchiness. The added group in the current study had a withdrawal rate of 11.5% because of adverse reactions, which included palpitations, chest pain, bradycardia, somnolentia, and ocular irritation. The difference in adverse effect-related subject dropout rate between our added and switched groups was not statistically significant. However, three patients from the added group and none from the switched group withdrew from the study. The subject age was significantly higher in the added group than in the switched group, that may have influenced this result. Lastly, a 12-week study examined the effects of switching from timolol to brimonidine in patients who were using PG analogue. A total of 1.9% of patients dropped out due to adverse reactions, which included ocular itchiness and somnolentia [21]. The patients who had experienced systemic or topical adverse events were 53.8% in added group and 40.7% in the changed group, which was equivalent between groups (P =0.4142). The switched group used PG/timolol FCs once daily and the added group used both a PG analogues (once daily) and 0.1% brimonidine (twice daily), therefore we predicted that the added group experience more adverse events. The number of subjects and short-term evaluation may also have influenced the results of this study. The IOP was measured after few hours of administration thus conjunctive hyperemia was not evaluated accurately.
Our study had several limitations. First, the number of subjects was small. Second, we only followed subjects for 3 months after medication changes were made. Therefore, this study only evaluated short-term IOP reduction efficacy and safety. Future studies should feature longer follow-up periods and also examine the effect of these medications on the preservation of visual function over the long-term.
CONCLUSION
In conclusion, this prospective study examined the safety and efficacy of two treatments in eyes of Japanese patients with POAG or OH. Both PG/timolol FCs and PG analogues plus brimonidine were equally safe and effective for lowering IOP. When PG analogues fail to sufficiently reduce Thus, PG with brimonidine might be appropriate medication in patients who cannot use PG/timolol FCs due to repiratory or circulatory disease. IOP, there was no difference between switching to PG/timolol FCs and adding brimonidine to PG analogue therapy. The patients’ adherence and systemic diseases should be considered.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.