All published articles of this journal are available on ScienceDirect.
Effect of Acute Increases in Intraocular Pressure on Corneal Pachymetry in Rabbit Eyes Treated with Timolol Maleate
Abstract
Objective:
To evaluate in an in vivo rabbit model, the effect of topical timolol maleate therapy on the central corneal thickness response to acute intraocular pressure increases.
Method:
In this prospective and interventional controlled study, the central corneal thickness and intraocular pressure were measured in vivo in 12 rabbit eyes treated with topical timolol maleate for 1 month and in 12 controls at baseline, and after the intraocular pressure (measured by direct cannulation of the anterior chamber) was increased to 15 and 30 mmHg using a forced saline infusion into the anterior chamber.
Results:
There were no significant differences in the basal central corneal thickness values (control group, 373.2±12.9 µm; study group, 377.5±19.2 µm, p=0.5) or the central corneal thickness values when the intraocular pressure was increased to 15 mmHg (control group, 335.2±14.3 µm; study group, 330.0±32.1 µm, p=0.6) and to 30 mmHg (study group, 318.8±25.3 µm; control group, 329.8±21.0 µm, p=0.3).
Conclusion:
Rabbit corneas treated with topical timolol maleate for 1 month did not show a strain response to acute intraocular pressure increases that differed from control eyes. This is in contrast to a previous finding in which rabbit eyes treated with prostaglandin analogues had a greater decrease in central corneal thickness in response to a sudden intraocular pressure increase compared with untreated corneas.
1. INTRODUCTION
Glaucoma, the second leading cause of visual loss wordwide [1], is an acquired optic neuropathy with progressive retinal ganglion cellular death that leads to irreversible visual field loss [2].
Increased Intraocular Pressure (IOP) is one of the most important risk factors for glaucoma development, and the only effective treatment is lowering of the IOP. Therefore, the accuracy of the IOP measurement is of paramount importance. Goldmann Applanation Tonometry (GAT) is the current gold standard for IOP measurement [3]. It is well known that some corneal characteristics, such as the Central Corneal Thickness (CCT), clearly affect the accuracy of the GAT IOP measurements [4]. In addition, corneal biomechanical properties, such as Young’s modulus of elasticity, might have a greater impact on IOP measurements than the CCT [5]. Furthermore, glaucoma can modify the cornea. The posterior corneal surface is more elevated in patients with glaucoma than in healthy subjects [6], lowering the IOP in steroid-induced glaucoma decreases the posterior corneal elevation and the CCT [7] and IOP elevation produces refractive changes in patients who underwent a previous corneal laser refractive surgery [8].
The corneal biomechanical properties determine the response of the corneal tissue when stress is exerted on the tissue [9]. There are different ways to analyze the corneal biomechanical behavior [10-12] and, recently, several devices designed to evaluate some of these properties in vivo, such as the Ocular Response Analyzer (ORA) (Reichert Technologies, Depew, NY) and the Corvis ST (Oculus, Arlington, WA), have become available to clinicians [13].
Some antiglaucoma drugs affect the cornea. Topical Carbonic Anhydrase Inhibitors (CAIs) might cause corneal edema when instilled on susceptible corneas [14], and these drugs seem to induce a slight increase in the CCT in normal corneas [15]. In an animal model, we found that topical CAIs affect the Intrastromal Corneal Pressure (ICP) [16], as do alpha-2 agonists, although to a much lesser degree [17].
However, Prostaglandin (PG) analogues induce a slight decrease in the CCT in humans [18] and affect the corneal biomechanical properties, i.e., topical use of these agents increases the Corneal Hysteresis (CH) [19]. In a previous study of our group, we showed that PG analogues modify the corneal strain response (decrease in CCT) in response to acute IOP increases in an animal model [2]. To further investigate if these biomechanical effects are related to the IOP-lowering effect of PGs or result from the direct effect of these drugs on the corneal collagen structure, we evaluated the effect of treatment with other topical antiglaucoma drugs, such as timolol maleate, on the corneal response to acute IOP increases in rabbit eyes.
2. MATERIAL AND METHODS
This was a prospective, interventional, controlled study. We recorded the IOP and increased the IOP level following exactly the same protocol that we described previously [2]. The experiments were performed in the eyes of male New Zealand rabbits (weight, 2.5-3.5 kg). The animals were treated according to the current regulations on experimentation and animal protection of the Government of Spain (RD 53/2013) and the recommendations of the Council of Europe agreement (ETS 123). Topical 0.5% timolol maleate (Timoftol, Merck Sharp & Dohme de España, S.A., Madrid, Spain) was instilled in the right eyes of 12 rabbits twice daily (morning and evening) for 1 month. Artificial tears (Acuolens, Alcon Cusí S.A., Barcelona, Spain) were instilled twice daily in the left control eyes of the rabbits. The investigators who performed the experiment did not know which rabbit eyes were treated with timolol maleate or artificial tears, so the measurements were obtained in a masked fashion. All rabbits were anesthetized with a mixture of ketamine 1 mL/kg (Imalgene, Merial Laboratory, Lyon, France), xylazine 0.75 mL/kg (Rompun, Bayer AG, Leverkusen, Germany), and intramuscular atropine. A topical anesthetic agent (Colicursí Anestesico Doble, Alcon Cusí) was instilled at the beginning of the experiment and supplemental doses were administered every 10 minutes in both eyes. The rabbits were euthanized after the experiment was completed.
The basal CCT and IOP values were recorded in both eyes after anesthesia was induced. The measurements were obtained using the DGH 550 Pachette 2 ultrasound pachymeter (DGH Technology, Inc., Exton, PA), and the average of 10 pachymetry measurements obtained automatically served as the CCT value for each eye. The basal IOP was measured using the Tono-Pen XL tonometer (Medtronic, Jacksonville, FL). The average of three consecutive measurements served as the basal IOP of each eye. The anterior chamber then was cannulated using a winged infusion set with a 27-gauge needle filled with 0.9% sodium chloride; the needle was inserted into the anterior chamber through the paralimbic sclera and cyanocrylate glue was applied to the entry point to ensure water tightness. The IOP was measured by manometry using a pressure transducer (MLT0390 Reusable BP transducer, Power Lab, AD Instruments, Racine, WI), which has a pressure measurement range from −80 to +380 mmHg and an accuracy of ±1 mmHg. The transducer is an external sensor for coupling to the IOP in the anterior chamber via a saline-filled silicone tube attached to the catheter (the winged infusion set), and it is connected to an amplifier (ML110 Bridge Amplifier, AD Instruments), allowing direct pressure measurement at the anterior chamber (Fig. 1). The transducer was prepared according to the manufacturer’s instructions to ensure a tight seal and that all air was flushed from the system. The recorder was set to 0 to initialize the transducer. Before starting the procedure, the transducer was checked to verify correct pressure registration. All instruments, tubes, and needles used in all experiments had exactly the same characteristics, including length and diameter. A second 21-gauge catheter was inserted into the anterior chamber and sealed in the same way. This cannula was connected to an external water column from which variable pressure increases could be induced. In this experiment, constant pressure was generated through a sphygmomanometer wrapped around a water reservoir to stabilize the IOP at 15 mmHg for 5 minutes and then at 30 mmHg for another 5 minutes. Before starting the experiment, a valve between the cannula and the water column blocked the water passage into the inner ocular space. The valve was opened and the pressure in the anterior chamber increased because of the water head.
The CCT measurements were performed again in both eyes of each rabbit 5 minutes after the anterior chamber pressure was increased to 15 mmHg and again 5 minutes after the IOP was increased to 30 mmHg. All eyes were instilled with a saline solution to prevent corneal drying throughout the experiment.
The right or the left eye of each rabbit was chosen randomly to begin the sequence of measurements. The same masked investigator recorded all measurements in the same manner during the afternoon (3 to 6 PM) in every eye. Statistical analysis was performed using the StatView SE + Graphics (Abacus Concepts Inc., Berkeley, CA) software on a Macintosh PowerBook 1400 cs/117 (Apple Computer Inc., Cupertino, CA) personal computer. The data are expressed as the average ± standard deviation and range. The nonparametric Mann-Whitney and Wilcoxon signed-rank tests were used for comparisons when appropriate. The exact p-value is expressed for each comparison. p < 0.05 was considered significant.
3. RESULTS
Measurements were obtained from the study eyes and control eyes of all 12 New Zealand rabbits. The mean basal IOP values before anterior chamber cannulation were 10.10 ± 3.6 and 12.0 ± 2.10 mmHg in the study and control eyes, respectively. The difference in basal IOP between the groups was not significant (p= 0.1).
The mean basal CCT values before anterior chamber cannulation were 377.5 ± 19.2 and 373.2 ± 12.9 µm in the study and control eyes, respectively, a difference that was not significant (p = 0.5). Five minutes after the anterior chamber pressure was stabilized at 15 mmHg, the CCT values decreased in both groups; the mean CCT decreased to 335.2 ± 14.3 and 330.0 ± 32.1 µm in the control and study eyes, respectively (p=0.002 for both comparisons). Five minutes after the IOP was stabilized at 30 mmHg, the mean CCT measurements decreased to 318.8 ± 25.3 and 329.8 ± 21.0 µm in the study and control eyes, respectively (No significant differences were seen in the mean CCT measurements between the study and control eyes at either IOP level (p = 0.6 and p = 0.3, respectively) (Fig. 2).
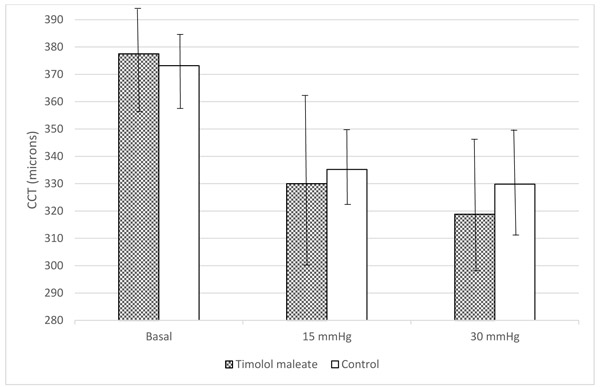
4. DISCUSSION
In the current study, we did not find a significant difference between groups in either the basal CCT or the CCT decrease in response to IOP increases to 15 and 30 mmHg.
The current results agreed with previous reports that do not find a change in CCT after treatment with timolol maleate. Viswanathan et al. [20] reported no significant reduction of the CCT in eyes treated with beta-blockers, and other studies reported no significant changes in the CCT after 6 and 12 months, respectively [21, 22]. Nevertheless, another study found a reversible increase in the CCT [23]. In that study, the maximal increase in the CCT after topical administration of beta-blockers occurred after 9 days, and afterwards the CCT returned to its basal value. Therefore, it makes sense that in our study in which we measured the CCT after 1 month of topical treatment that we found no change in the CCT in study eyes compared with control eyes.
However, we found that the CCT decreased when the IOP increased and that the higher the IOP value the greater the CCT decrease was [2]. This agreed with the results of other studies [7]. The findings of the current study suggest that when the IOP increases, the stromal lamellae are compressed, probably because some fluid is expressed out of the corneal stroma [24]. Another explanation for the IOP-related decrease in the CCT could be that some degree of dehydration might have occurred during the experiment. Nevertheless, topical lubricant eye drops were used, and both eyes of each rabbit were randomized to be the first or the last to be examined, makes this potential confounding factor unlikely to bias our results.
The current study did not find a significant difference between both groups in the responses of the CCT to IOP increases. These results differ from those obtained previously in which we used the same study design but instilled PG analogues instead of timolol [2]. In another previous study, we observed that corneas treated with travoprost had a greater IOP-induced decrease in CCT from baseline than the control eyes. A possible explanation for this different behavior could have been the lower hypotensive effect of timolol than PG analogues [14], but we do not believe this is the case given the different response to hypotensive drugs in rabbit eyes compared with humans. In fact, the IOPs were similar in the current timolol maleate-treated eyes and the travoprost-treated eyes in the previous study (10.0±3.6 mmHg and 10.50±0.83 mmHg, respectively), so it seems unlikely that differences in the basal IOP could have affected the corneal response.
A possible explanation that could explain the different behavior of timolol-treated eyes compared with PG-treated eyes is that PGs not only reduce the IOP but also stimulate the local synthesis of Matrix Metalloproteinases (MMPs) in treated eyes [24]. The MMPs are a family of enzymes that degrade collagen types I, II, III, and IV [25, 26]. Considering that the corneal stroma has collagen types I, II, IV, and V [27-29] it is reasonable in our minds to consider that the corneal stroma might become less rigid after treatment with these drugs. However, topical beta-blockers that lower the IOP and reduce aqueous humour production by the ciliary epithelium [23, 30, 31] would not directly affect the corneal stroma, so the degree of corneal compression induced by a sudden IOP increase would remain unchanged. Thus, a plausible explanation for the current findings is that up-regulation of MMPs in the corneal stroma caused by PG analogues might directly affect the corneal biomechanical behaviour [13] that is absent after timolol treatment.
Evaluation of the corneal biomechanical properties is becoming increasingly important in glaucoma. Different methods are used to analyze the corneal biomechanical behaviour [32-34]. Analysis of the corneal strain (thickness change) in response to a sudden IOP increase is a sensitive method to detect differences between the different responses of the corneal stromal bed and the laser in situ keratomileusis flap in enucleated human eyes [35]. Other methods are more suitable for measuring the corneal biomechanical properties in vivo, such as the ORA, which provides the CH and the corneal resistance factor, which represent the corneal viscoelastic response to air. The evaluations of the changes in CH after PG analogue treatment in humans are consistent with the current findings. We found that PG-treated corneas increase the CH, which is unrelated to the drug-induced IOP decrease, thus further supporting the hypothesis that PG analogue treatment directly affects the corneal biomechanical behavior independent on the hypotensive effect [36]. The clinical relevance of this finding is that it might well be that treatment with PG analogues may be an artifact for tonometry, and thus there is the theoretical possibility that the hypotensive effect of these drugs may be over or underestimated. It is evident that this is just a speculation that needs further specifically designed studies to be tested.
A limitation of the current study was that the results in animals are not easily directly applicable to humans. Nevertheless, we believe that the finding that lowering the IOP using topical hypotensive drugs per se does not affect the biomechanical behaviour of the cornea unless the drug used has some direct effect on the collagen is correct, at least in rabbits. It is evident that only studies performed in humans can verify if the human cornea shows the same type of response than the rabbit one.
CONCLUSION
In summary, rabbit corneas treated with topical timolol maleate do not show a strain response to acute IOP increases that differs from that in control eyes. This is in contrast with the previously reported [2] finding that rabbit corneas treated with PG analogues have a greater decrease in CCT in response to a sudden IOP increase. Our findings suggested that PGs may induce changes in the corneal biomechanical properties independent of their hypotensive IOP, at least in rabbits.
Further studies are needed to clarify the possible changes in the strain properties of the cornea induced by antiglaucomatous therapy and the consequences that these changes might have on IOP measurements in these eyes and to determine if our findings are applicable to other species, such as humans.
LIST OF ABBREVIATIONS
| IOP | = Intraocular Pressure |
| GAT | = Goldmann Applanation Tonometry |
| CCT | = Central Corneal Thickness |
| ORA | = Ocular Response Analyzer |
| CAIs | = Carbonic Anhydrase Inhibitors |
| ICP | = Intrastomal Corneal Pressure |
| PG | = Prostaglandin |
| CH | = Corneal Hysteresis |
| MMPs | = Metalloproteinases |
FUNDING
Supported in part by Grant number 11-01 from Fundación para la Investigación Biomédica del Hospital Universitario Príncipe de Asturias.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board, Comité de Ética en Investigación Clínica of the “Hospital Universitario Príncipe de Asturias.
HUMAN AND ANIMAL RIGHTS
No Humans were used in this research. All animal research procedures followed were in accordance with the current regulations on experimentation and animal protection of the Government of Spain (RD 53/2013) and the recommendations of the Council of Europe agreement (ETS 123).
CONSENT OF PUBLICATION
Not applicable.
CONFLICT OF INTERESTS
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
ACKNOWLEDGEMENTS
Cristina Sanchez Barahona: Performed study, Collected data, wrote paper.
Gema Bolívar: Designed study, analyzed data, wrote paper.
Dimitrios G. Mikropoulos: Analyzed data, wrote paper.
Anastasios G. Konstas: Analyzed data, wrote paper.
Miguel A. Teus: Design study, analyzed data, wrote paper.


