RESEARCH ARTICLE
Efficacy and Safety of an Intracameral Combination of Two Mydriatics and an Anesthetic for Phacoemulsification in Complicated Patients
Raffaele Nuzzi1, *, Valentina Baratozzi2, Maria Sole Polito1, Federico Tridico1
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 322
Last Page: 329
Publisher ID: TOOPHTJ-12-322
DOI: 10.2174/1874364101812010322
Article History:
Received Date: 16/09/2018Revision Received Date: 05/11/2018
Acceptance Date: 05/12/2018
Electronic publication date: 31/12/2018
Collection year: 2018

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Advantages of intracameral mydriatics have been demonstrated in healthy patients, but safety and efficacy in complicated subjects remain to be assessed.
Objective:
The purpose of this study is to evaluate efficacy and safety of an intracameral combination of phenylephrine (0.31%), tropicamide (0.02%) and lidocaine (1%) (Mydrane®, Thea Inc.) in phacoemulsification surgery in subgroups of patients affected by different systemic and ocular diseases.
Methods:
125 patients were recruited and compared with a control group of 39 patients. Both groups have been divided according to the presence/absence of ocular or systemic diseases. In course of surgery, grade of mydriasis and ocular analgesia have been evaluated by the surgeon. During follow-up, eventual adverse events have been monitored. Also, comfort reported by patients and surgeon has been investigated.
Results:
99.2% of patients receiving the intracameral formulation achieved acceptable mydriasis (> 6 mm), maintained during capsulorhexis, phacoemulsification and IOL insertion without the need of additional mydriatics. No adverse events or sings of unsuccessful surgery were observed among treated patients.
Conclusion:
An intracameral mydriatic solution can be a safe and comfortable tool for inducing and maintaining intraoperative mydriasis and analgesia, even in complicated patients.
1. INTRODUCTION
Currently, the cataract has a relevant socio-economic impact, if we consider its prevalence and the progressive increase in incidence , along with the European population ageing. Thus, an optimised cataract surgery should reduce the impact on healthcare resources.
Of course, micro-incisional phacoemulsification allowed reduced surgical time, post-operative recovery and hospitalization [1]. Optimal mydriasis and anesthesia still remain key points influencing the safety of surgery and patients’ comfort.
Major advantages of intracameral mydriatics in comparison with the topical regimen (which, in many cases, needs multiple administrations in order to achieve adequate effects) have been already demonstrated: single-use, limited ocular surface toxicity and decreased incidence of cardiovascular side effects [1].
Some pilot studies have reported safety and efficacy of various ad hoc intracameral formulations for cataract surgery, prepared “on site”, containing phenylephrine and cyclopentolate or tropicamide [2].
In 2015 a controlled clinical trial evaluated the effects of a standardised intracameral mydriatic solution (Mydrane, Laboratoires Théa, Clermont-Ferrand, France) - which is the first ready-to-use injectable solution for intracameral administration of two mydriatics and an anesthetic – in comparison with the topical regimen. This study showed that the intracameral mydriatic is an efficient and safe method for inducing and maintaining intraoperative mydriasis and analgesia, leading to increased patients’ comfort (especially during IOL implantation) and surgeon satisfaction, as well [3]. Further benefits of standardized intracameral preparations are represented by prevention of drug-related errors and accordance to industrial quality checks.
In this observational prospective study, we investigated the efficacy and safety of an intracameral combination of phenylephrine (0.31%), tropicamide (0.02%) and lidocaine (1%) (Mydrane) during phacoemulsification and IOL implantation procedures - in comparison with topical mydriatic drugs - in patients affected by systemic or ocular diseases other than cataract.
2. MATERIALS AND METHODS
This study was conducted from July 2016 to November 2017 at two ophthalmic centres in Piedmont: The University Hospital San Luigi Gonzaga in Orbassano and the Ophthalmic Hospital in Turin. All procedures in this study concerning it's conduction and documentation were performed in conformity with the ethical principles set out in the Helsinki Declaration and its revisions. The intracameral mydriatic featured a salt-balanced and pH-balanced solution composed by two mydriatics (tropicamide 0.02% and phenylephrine 0.31%) and one anesthetic agent (lidocaine 1%). The standard topical regimen used in the control group featured an ocular insert with a combination of phenylephrine 5.4 mg and tropicamide 0.28 mg (Mydriasert, Laboratoires Théa, Clermont-Ferrand, France).
We recruited patients eligible for phacoemulsiphication with IOL implantation between 53 and 90 years old. At the baseline visit a pupil diameter of at least 6 mm had to be obtained 30 minutes after the instillation of phenylephrine 10% eye drops. At this selection visit patients’ data were gathered, including past medical and ophthalmological history. Participants were then divided into three different groups: individuals without any complicating disease, patients with systemic pathologies (in particular Type-2 diabetes, hypertension and benign prostatic hyperplasia) and patients revealing ocular pathologies at the time of the baseline examination – such as increased intraocular pressure, glaucoma, pseudoexfoliation syndrome, dense or total cataract and Fuchs' corneal endothelial dystrophy. The exclusion criteria were: mydriasis < 6mm, history of ocular trauma, and congenital cataract. Informed consent has been collected in written form for all participants. This study received the approval from the institutional Ethics Committee. The selected patients underwent cataract surgery with phacoemulsification technique and IOL implantation.
Also, patients that needed an additional intraoperative mydriatic treatment before capsulorhexis, presenting intraoperative complications related to the surgical procedure have been excluded from the study (Fig. 1).
All surgical procedures were performed by a single surgeon with the same technique. Pupil diameter was measured by the same surgeon with a surgical ruler and/or a surgical compass in three specific stages of the procedure: right before corneal incision (T0), before viscoelastic injection (T1), before capsulorhexis (T2).
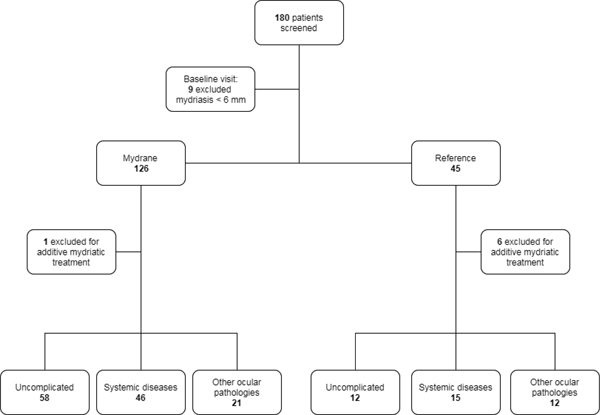 |
Fig. (1). Patient selection procedure and distribution of the study population. |
Follow-up examinations were scheduled at one day and one week postoperatively.
The primary efficacy outcome was capsulorhexis realization without the need of any additional mydriatic drug, or mechanical device. Secondary efficacy outcome was achievement of an acceptable mydriasis (pupil diameter of at least 6 mm) [4-6] just before capsulorhexis.
Main postoperative outcomes included: Intraocular pressure, miosis recovery, presence of lens residues and anterior chamber reactivity. Observation for possible local and systemic adverse events has been performed in the groups under study, during each follow-up visits. Furthermore, for each procedure, patient comfort and surgeon satisfaction have been reported.
Statistical significance of differences between groups was calculated with Student’s T test for non-parametric variables. Descriptive statistics were calculated for quantitative variables using the test F based on Fisher-Snedecor distribution. Differences between groups were tested with a two-sided 95% Confidence Interval (CI).
3. RESULTS
Of 126 selected patients in the intracameral mydriatic group, 125 achieved an acceptable mydriasis, according to the primary efficacy criteria: only one subject needed an additional mydriatic drug (adrenaline).
Capsulorhexis was successfully performed in 99.2% of patients from the intracameral mydriasis group. None of the study participants presented intraoperative complications related to surgical procedure.
Of the 45 selected patients that received the topical treatment, 39 (92.86% of intention-to-treat) were included in the control group, since 6 did not respect the primary efficacy criteria. Baseline patients’ characteristics were similar between the two groups (Tables 1-2).
| Intracameral Mydriatic Group (n = 125) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Uncomplicated | n | % | Systemic Disease | n | % | Others Ocular Pathologies | n | % |
| Total | 58 | 100 | - | 46 | 100 | - | 21 | 100 |
| Male | 18 | 31 | Male | 25 | 54 | Male | 10 | 48 |
| Female | 40 | 69 | Female | 21 | 46 | Female | 11 | 52 |
| Age (mean) | 75 | - | Age (mean) | 75 | - | Age (mean) | 77 | - |
| Age (median) | 76 | - | Age (median) | 75 | - | Age (median) | 77 | - |
| Age (mode) | 79 | - | Age (mode) | 72 | - | Age (mode) | 77 | - |
| Age (range) | 58-87 | - | Age (range) | 59-80 | - | Age (range) | 56-95 | - |
| Age (SD) | 6,09 | - | Age (SD) | 6,12 | - | Age (SD) | 7,98 | - |
| Light Iris | 17 | 29 | Light Iris | 18 | 39 | Light Iris | 7 | 33 |
| Dark Iris | 41 | 71 | Dark Iris | 28 | 61 | Dark Iris | 14 | 67 |
| Control Group (n = 39) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Uncomplicated | n | % | Systemic Disease | n | % | Others Ocular Pathologies | n | % |
| Total | 12 | 100 | - | 15 | 100 | - | 12 | 100 |
| Male | 3 | 25 | Male | 3 | 20 | Male | 9 | 75 |
| Female | 9 | 75 | Female | 12 | 80 | Female | 3 | 25 |
| Age (mean) | 66 | - | Age (mean) | 73 | - | Age (mean) | 80 | - |
| Age (median) | 65 | - | Age (median) | 75 | - | Age (median) | 79 | - |
| Age (mode) | 53 | - | Age (mode) | 70 | - | Age (mode) | 79 | - |
| Age (range) | 53-83 | - | Age (range) | 60-82 | - | Age (range) | 70-93 | - |
| Age (SD) | 12,52 | - | Age (SD) | 8,17 | - | Age (SD) | 8,59 | - |
| Light Iris | 3 | 25 | Light Iris | 6 | 40 | Light Iris | 3 | 25 |
| Dark Iris | 9 | 75 | Dark Iris | 9 | 60 | Dark Iris | 9 | 75 |
Moreover, the response rate (defined by a pupil size of at least 6 mm measured just before capsulorhexis) was 80.8% in the intracameral regimen group and 53.8% in the control group. In both groups age, sex and iris color did not induced differences in mydriasis either before or in course of surgery.
A deepened analysis has been conducted into the two groups to evaluate different responses in three subgroups according to any clinical risk due to different pathologies. Responding rates (pupil diameter >6 mm at T2) in the intracameral mydriatic group showed best results in patients unaffected by ocular or systemic comorbidities. On the contrary, individuals treated with topical regimen and affected by systemic diseases revealed a higher response rate, as shown in Table 3. Whereas, no patient in the ocular comorbidities-subgroup achieved an adequate mydriasis, even if the surgery has been carried out anyways.
| Intracameral Mydriatic Group: 125 patients | |||
|---|---|---|---|
| - | Uncomplicated | Systemic Disease | Others Ocular Pathologies |
| N | 58 | 46 | 21 |
| Responder | 53 | 32 | 16 |
| % | 91,3 | 69,5 | 76,2 |
| Control Group: 39 patients | |||
| - | Uncomplicated | Systemic Disease | Others Ocular Pathologies |
| N | 12 | 15 | 12 |
| responder | 9 | 12 | 0 |
| % | 75 | 80 | 0 |
Higher non-response indicators according to secondary efficacy outcome has been observed in the subgroup of patients with systemic comorbidities: 80% (p-value = 0,03) based on a logistic regression model adjusted by sex, age and iris color variables. Furthermore, a statistically significant difference in pupil diameter at both times T1 (p-value = 0,002) and T2 (p-value = 0,02) has been reported. In fact, complicated patients presented a smaller pupil diameter (particularly in cases of concomitant α-lithic intake, with a reduced mydriasis mostly right before viscoelastic insertion; p-value = 0,007). Also, patients affected by ocular pathologies presented a smaller diameter in comparison with unaffected participants, but only at T2 (p value = 0,043).
Differences in pupil size observed in subgroups treated with topical regimen showed to be statistically significant when comparing people without comorbidities and patients with ocular morbidities at T0 (p value = 0,02) and T2 (p value 0,01). In addition, even subgroups of individuals affected by systemic diseases presented better pupil dilation in all three surgical times in respect with the one with ocular diseases at T0 (p value = 0,001), T1 (p value = 0,008), and T2 (p value = 0,002). We didn’t observe significant differences when comparing non-complicated patients and patients with systemic diseases.
Postoperative safety assessments included ocular symptoms and slit lamp examination, measurement of intraocular pressure (including mean IOP and cases of ocular hypertension, OHT, defined as IOP>21 mmHg), evaluation of anterior chamber, pupil shape and size recovery at one day and one week after surgery.
The only statistically significant difference between the various groups was observed in IOP values at both one day and one week postoperatively with higher values and more frequent cases of ocular hypertension among complicated patients receiving the intracameral solution, when compared with healthier individuals. Among patients receiving the intracameral solution the group with systemic comorbidities presented a mean IOP of 18 mmHg and 13 cases of OHT at 1 day after surgery and mean IOP of 16 mmHg and 2 cases of OHT at 7 days; patients affected by ocular comorbidities showed a mean IOP of 17 mmHg and 7 cases of OHT at 1 day and mean IOP of 16 mmHg and 4 OHT cases at one week postoperatively. Alternatively, healthy patients receiving the intracameral treatment presented mean IOP values of 17 mmHg with 8 cases of OHT at one day after intervention, while at one-week follow-up they showed a mean IOP of 15 mmHg with 2 cases of OHT.
In consideration of this outcome, we have constituted a regression logistic model to evaluate the evolution of risk over time, adjusted for age, sex and iris color. Regarding the study group, we detected a lower risk of increased IOP at one week after surgery (decreased from 22% to 6%). In particular, the IOP trend improved in the subgroup of patients without comorbidities.
Statistical analysis regarding anterior chamber examination and pupil size didn’t show significant difference in various subgroups. No additional ocular adverse events were observed in the treatment group after surgery.
4. DISCUSSION
Mydrane is the first industrially standardized mixture of mydriatics and anesthetic for injection in the anterior chamber designed for phacoemulsification surgery. A previous randomized clinical trial demonstrated that intracameral administration of Mydrane just after the first incision produces rapid and adequate mydriasis in non-complicated patients, allowing phacoemulsification in regular conditions, showing similar efficacy with the standard topical regimen [3]. The active components, concentrations and volumes in the final formulation were based on the efficacy and safety of other intracameral formulations used for phacoemulsification in cataract surgery [1, 2]. Moreover, several studies on animal models reported the tolerability at the level of the corneal endothelium of intracameral solutions of phenylephrine, tropicamide and lidocaine, even as a fixed combination [7-9].
Intracameral administration after corneal incision can provide a considerable reduction in preoperative preparation without significant increase in the duration of surgery. Preoperative time was much lower in the intracameral mydriatic group as there was no requirement for topical drops preoperatively. A reduction in time spent in the preoperative room can also lead to a less stressful experience for patients, which has been confirmed by patients receiving the intracameral solution.
In this observational study, the non-inferiority of the mydriatic solution under evaluation was thus demonstrated, since mydriasis without any concomitant pupil-expanding treatments has been achieved in a higher percentage, if compared with the control group. In addition, response rate in the study group, with a pupil size of at least 6 mm just prior to capsulorhexis, was very high (80.8%) and greater than the reference group (53.8%). Splitting the study group was useful to highlight the best response rate in healthy individuals (91.3%) - as a confirmation of previous studies – and a worse one in complicated patients, although still adequate enough to perform surgery without technical difficulties (systemic diseases group 69.5%, ocular diseases group 76.2%). In these complicated cases, a better mydriasis can also be obtained using a suitable viscoelastic (with different molecular weights, according to a case-by-case evaluation).
In the control group the highest response rate was reported in patients with systemic pathologies (80%), whereas the 75% of healthy patients achieved an adequate mydriasis and no participants with ocular comorbidities obtained a mydriasis superior to 6 mm.
From the analysis made on the group receiving the intracameral solution, healthy patients turned out to have greater mydriasis than participants with systemic diseases during surgery at both T1 (viscoelastic injection) and T2 (just before phacoemulsiphication). According to the previous literature, diabetes causes neuropathy and vascular alterations which compromise pupillary dilation [10, 11]. Moreover, therapies for hypertension and benign prostatic hypertrophy with alpha-adrenergic receptors antagonists invalidate the activity of pupil dilating muscle [12]. However, significant differences have not been observed in the reference group of this study.
A reduced mydriasis in ocular-complicated patients in comparison with the healthier patients has been reported in both study (with statistical significance at T2) and control group (at T0, T1, T2). This could be explained by more likely inflammatory reactions of the ocular anterior segment which, if added to the senile reduced activity of intrinsic ocular muscles, induce a poor mydriasis [13]. Pharmacologic mydriasis in these cases can be improved by slow intracameral injection of mydriatic solutions and by using a high-molecular-weight viscoelastic. The correct IOL placement was achieved in all patients, in both groups.
To evaluate treatment safety, we have considered postoperative parameters at one day and one week after surgery. Prevention of sustained postoperative IOP is considered extremely important to avoid macular edema [14]. At one day after surgery, the frequency of an Increased Ocular Pressure (IOP) in the study group was smaller for healthy patients (14%), in comparison with the systemic-complicated (28%) or ocular-complicated ones (33%). Similarly, after one week, the rates were higher for people affected by systemic diseases. In the subgroup of patients affected by ocular pathologies this result was however expected since one third of its components had glaucoma or increased IOP at the registration visit. Miosis recovery has been achieved in all patients postoperatively, and no significant differences have been observed in all groups.
Additionally, the presence of lidocaine 1% in the intracameral solution led to improved intraoperative anesthesia, with lower patient discomfort during IOL insertion, when compared with the standard topical regimen. Moreover, the surgeon reported a higher satisfaction, related especially to IOL insertion steps in the study group.
CONCLUSION
The intracameral solution of phenylephrine, tropicamide and lidocaine can be safely used in complicated patients and eyes, with reduced preparation time, allowing a complete mydriasis management by the sole surgeon. This technique is not inferior when compared with traditional mydriatic agents, also in complicated patients.
The intraoperative mydriasis management can lead to improvements in the whole operative setting, and a reduced preoperative instillation of mydriatics provides a lesser stressful experience for the patients and a complete control of all surgical aspects by the surgeon, which can avoid the application of mechanical dilating devices (with reduced damage on the iris tissue). This method can also influence ocular analgesia, following intracameral anesthetic injection, which (when associated with mydriatic agents) reduces the number of needed products and preparatory steps, with lower risk of administration errors, total management time and costs.
LIST OF ABBREVIATION
| IOL | = Intraocular Lens |
| CI | = Confidence Interval |
| IOP | = Intraocular Pressure |
| OHT | = Ocular Hypertension |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study has been approved by the Institutional Ethics Committee of Institute of Ophthalmology.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
CONSENT FOR PUBLICATION
Informed consent to participate has been collected in written form from all participants.
CONFLICT OF INTERESTS
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
RN gave a major contribution to the conception and realization of this study, performed cataract surgery interventions on each patient enrolled in this study and administered intracameral mydriatic solution. RN, VB, MSP and FT gave a major contribution for patient enrollment, ocular and functional examination and follow-up, analyzing and interpreting patient data and giving their contribution in writing the manuscript.







