All published articles of this journal are available on ScienceDirect.
Fast Measure of Visual Acuity and Contrast Sensitivity Defocus Curves with an iPad Application
Abstract
Objective:
To evaluate the repeatability of the fast measurement of the visual acuity (VADC) and contrast sensitivity (CSDC) defocus curves with a new test as well as the agreement of measurements at far distance obtained with the Early Treatment Diabetic Retinopathy Study (ETDRS) chart and the ClinicCSF test for measuring Contrast Sensitivity Function (CSF).
Method:
Records from fifty-nine subjects implanted with Multifocal Intraocular Lenses (MIOLs) were retrieved from our database. VADC and CSDC were measured from +1.00 D to -4.00 D in 0.50 D steps. The agreement with the ETDRS and the CSF at far distance was assessed in comparison to the 0 D location of the VADC and the CSDC, respectively. The repeatability was evaluated in 34 subjects who consecutively repeated two measures.
Results:
Median Visual Acuity (VA) was -0.1 logMAR with the VADC at 0 D of defocus and 0 logMAR with the ETDRS (p>0.05). A total of 45.8% of eyes showed no differences between both tests and the difference was less than one line of VA in 96.6% of the eyes. The intrasubject repeatability was under one line of VA along all the defocus curve except for positive defocus levels. The CSDC showed the best agreement with the CSF for 18 cycles per degree. The CSDC was less repeatable than VADC. Mean time spent on completing the VADC and CSDC was 7.81 and 7.98 minutes, respectively.
Conclusion:
The VADC showed good agreement with the ETDRS and good repeatability despite the short testing time. In contrast, poorer repeatability was found for CSDC. Our method would facilitate the inclusion of VADC in clinical practice as it is a fast test, being also the first one including the measure of CSDC.
1. INTRODUCTION
Depth of field is defined as the distance in the object space within which the image of an object has an acceptable sharpness [1]. Many optical compensations and surgical procedures are focused on strategies for increasing depth of field and consequently improving vision at certain distances. Examples of these techniques are multifocal contact lenses, PresbyLASIK procedures, and the replacement of the lens by a Multifocal Intraocular Lens (MIOL) in cataract surgery or refractive lens exchange [2-4]. The measure of the depth of field, defined as the range of vision above a particular value of visual acuity, [5] should not be confused with the measure of visual performance through the field. Whereas the first one requires only to bring in and out a target to find the blur limits, the second one requires to look for the threshold of vision at each particular distance and therefore it requires longer testing times.
The most popular method in clinical practice for evaluating the visual performance through the field with a MIOL is the Visual Acuity Defocus Curve (VADC) [6], which consists of testing Visual Acuity (VA) through different levels of spectacle lens defocus with a VA chart. Despite several charts are available in clinical practice for testing VA, the Early Treatment Diabetic Retinopathy Study (ETDRS) VA chart is considered the gold standard and it is used in clinical trials with MIOLs [7]. However, ETDRS is usually conducted with static charts which require a long-time testing process, presenting the defocus lenses in a random order, or changing the chart during the process for avoiding memorization [8, 9]. For these reasons, VADC is not widely included in clinical practice and it is recommended to use digitized charts with the randomized presentation of test letters for not requiring defocus lens randomization [7].
VADC has also the limitation of not being very sensitive to little changes in optical quality [10]. For this reason, low contrast VA tests [11], Contrast Sensitivity (CS) tests based on letters [12], and sinusoidal gratings [13] have been commonly used for assessing visual performance with MIOLs. CS is less repeatable than VA [14] and therefore requires complex psychophysical methods to obtain reliable measures, taking between 2 to 5 minutes per defocus point for the fastest reported procedures [14, 15]. This means that for testing a CSDC with 11 defocus points, time spent would be around 22 and 55 minutes per eye. The main aim of this study was to introduce a new iPad app designed for the fast measure of VADC and CSDC. The first aim was to evaluate the agreement between the optotype used, crowded Snellen E versus the ETDRS for VA, and crowded Snellen E versus sinusoidal gratings from the ClinicCSF [10] test for measuring CSF. Then, the repeatability of the new app was evaluated for a range from +1.00 to -4.00 D of defocus lenses in -0.50 D steps.
2. MATERIAL AND METHODS
2.1. Subjects and Procedures
Data for the experiments were retrospectively retrieved from the postoperative visits of subjects implanted with trifocals and bifocal IOLs. These IOLs are not detailed because the main purpose of the study was not related to the IOLs but was related to the agreement between tests and the inclusion criteria were not limited to any particular model. Only one eye from each individual was selected randomly and included in the analysis. Exclusion criteria were any ocular disease or surgery complication reported in the clinical history that might affect the visual performance such as capsular tears or capsular opacification, among others. Manifest subjective refraction was performed at a distance of 2 m with the ETDRS chart for iPad previous to any visual performance measurement. An additional +0.50 D was inserted in the trial frame during the subjective refraction for correcting vergence distance. This additional lens virtually moved the location of the object from 2 m to infinite ensuring that the point of highest VA in the defocus curve was located at 0 D. Therefore, all the tests were conducted at 2 m with the subject wearing the best spectacle correction and an additional +0.50 D lens. The presentation time for each stimulus in all the tests was not limited by the device, giving to the patient the time needed for taking a decision. This prospective observational study was approved by the local ethics committee of research and was performed in adherence to the tenets of the Declaration of Helsinki.
2.2. Defocus Curve Application Test
The defocus curve app (version 1.0.8) was developed by pure mobile ActionScript 3.0 code and compiled for IOS with Adobe Flash Builder (Adobe Systems, Inc.) for being reproduced in an iPad with at least 264 points per inch as the used in this study (model A1458, Apple Inc., Cupertino, CA, USA). The screen background brightness was set to 85%, ~250 cd/m2. The app was designed to measure VADC and CSDC using a Snellen E crowded optotype that changed its size or contrast depending on the selected procedure. We used the Snellen E because this has been employed for theoretically describing the relationship between spatial frequency with gratings and the VA [16]. For VADC, a high contrast optotype (100%) changed its size along the range from 1.0 to -0.2 logMAR in 0.1 logMAR steps, whereas for CSDC, a fixed size optotype, equivalent to 0.3 logMAR size, changed its contrast along the range from 0 to 1.9 logarithmic units of CS (logCS) in 0.1 logCS steps (Fig. 1A). The bitStealing method was used for expanding the capabilities of the display to represent contrasts [17], and the gamma correction obtained from the mean of different displays was used for implementing this method [18].
The measurement procedure, either for VADC or CSDC, started with an alert message that indicated the defocus lens that should be inserted (starting in +1.00 D). The experimenter explained to the subject to respond one of the four possible orientations of the Snellen E in such a way that the experimenter only had to press the button corresponding to the answer given by the subject. The orientation of the Snellen E after each answer was presented randomly. The size (VADC) or contrast (CSDC) changed according to a simple up-down staircase psychophysical method which ended after three reversals [17], obtaining the threshold with the average of these three reversals [10]. After testing the threshold for the first defocus lens, a new alert appeared over the screen with the defocus lens which should replace the previous one (from +1.00 D to +0.50 D) and the three reversals were repeated but then starting at the VA or CS threshold obtained with the previous defocus lens. This procedure was repeated for all the defocus lenses from +1.00 D to -4.00 D, in -0.50 D steps. The total time spent by the procedure was automatically recorded by the app, detecting the time that the experimenter needed to change the defocus lens during the alert messages and the time that the patient needed to answer to the optotypes orientation.
2.3. Conventional Tests Description
For visual acuity, the agreement at a far distance with the Snellen E was evaluated versus an ETDRS chart. The ETDRS chart (Fig. 1B) was reproduced in the iPad at 85% of screen brightness which corresponded to 250 cd/m2, spectral centroid 542 nm (measured with a Spyder4Elite colorimeter) [19]. The procedure for obtaining the VA threshold with the ETDRS chart included the following steps [20]: 1) the subject read the first letter of the starting row and advanced one row with each right answer reading only one letter per row, 2) after the first mistake, the subject went to the previous row and read it completely, 3) advancing to the next row if the subject read 3/5 letters or went back to the previous if read less than 3/5; 4) the threshold was the last line for which the subject read 3/5 letters.
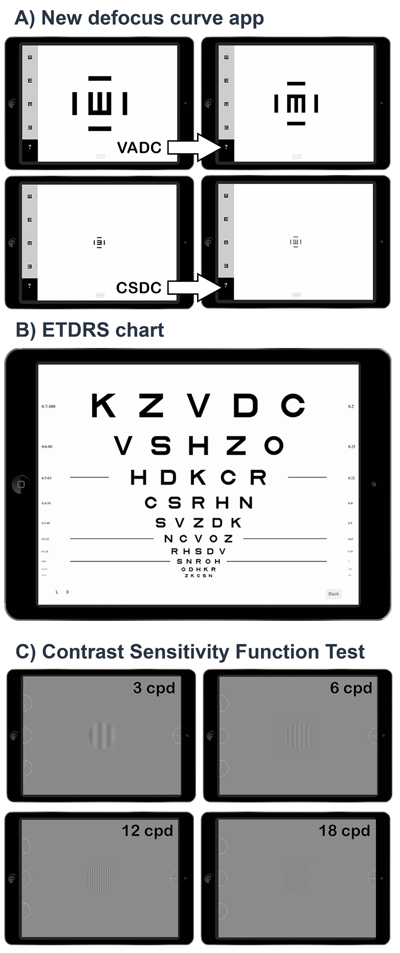
For contrast sensitivity, the agreement at far distance with the Snellen E was evaluated versus a CSF test previously validated (ClinicCSF) [10, 14], consisting of four sinusoidal gratings (3, 6, 12 and 18 cycles per degree, cpd) over a mean achromatic background brightness of 85 cd/m2 (Fig. 1C). Nine patches of different contrast levels were presented to the subject for each spatial frequency following a simple up-down staircase psychophysical method [17], starting in the fifth patch level for each spatial frequency. In this method, CS went one level up (e.g. from level 5 to 6) after each right answer until the observer failed. Then, CS went down until the observer get right again. The CS threshold was determined by averaging the sensitivities at the turnaround points (i.e. the CS at the levels where direction changed) in the adaptive track for a total of five reversals.
2.4. Experiment 1. Agreement at a Far Distance
Data of fifty-nine subjects (44 women and 15 men) were included in the agreement experiment for far distance. The VA was first measured with the ETDRS chart after achieving the best spectacle refraction. Then, the CSF was tested with the CSF test, including spatial frequencies of 3, 6, 12 and 18 cpd. Finally, the VADC and CSDC were measured for the same eye in a random order. The agreement for VA between tests was obtained comparing the ETDRS with the 0 D defocus lens resulting from the VADC, whereas for CS, the four spatial frequencies measured with the CSF test were compared with the same defocus (0 D) of the CSDC.
2.5. Experiment 2. Repeatability Along the Defocus Curve
Data of sixty-eight subjects (46 men and 22 women) were included in the repeatability experiment, thirty-four subjects in the repeatability analysis of the VADC and another thirty-four in the repeatability analysis of the CSDC. The measurement conditions were the same detailed previously and two monocular defocus curves were obtained consecutively in each patient for an eye selected randomly.
2.6. Statistical Analysis
Normal distribution for differences between tests in the agreement experiment was tested with the Kolmogorov-Smirnov test. The Wilcoxon test was used for testing median differences in paired comparisons (z) and the Spearman’s rho (ρ) for correlations because of the non-normally distributions. The repeatability was calculated with one-way Analysis of Variance (ANOVA) and the repeatability limit (R) was equal to 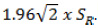 , [21]. The non-parametric Passing–Bablok model of linear regression was used to assess the agreement, as the Bland and Altman method cannot be used with non-parametric data samples [22]. The statistical analyses were performed using the IBM SPSS 20.0 software for Windows (SPSS, Chicago, IL) and MedCalc Software 15.2 (MedCalc, Ostende, Belgium).
, [21]. The non-parametric Passing–Bablok model of linear regression was used to assess the agreement, as the Bland and Altman method cannot be used with non-parametric data samples [22]. The statistical analyses were performed using the IBM SPSS 20.0 software for Windows (SPSS, Chicago, IL) and MedCalc Software 15.2 (MedCalc, Ostende, Belgium).
3. RESULTS
3.1. Experiment 1. Agreement at a Far Distance
Mean subject’s age was 59.7 ± 8.5, ranging from 40 to 77 years old. There were no significant differences between both procedures z = .66, P = .51 for measuring VA at a far distance (Table 1).
A total of 45.8% of cases matched the same value of VA, 25.4% overestimated 1 line, and 25.4% underestimated 1 line with the new app. Therefore, 96.6% of eyes showed a difference equal of less than one line between methods, with only two cases (3.4%) showing an overestimation of 2 lines with the new app. The Passing Bablok analysis showed no systematic or proportional differences between VA tests. Similar correlations were found between visual acuities and age (rho = 0.40, P = 0.002 with the new app and rho = 0.42, P = 0.002 with ETDRS).
The logCS measured with the new app was significantly different for all the tested spatial frequencies compared to the CSF (Table 1), the difference between tests decreased with the increment of the spatial frequency. Correlations were found between CS at all the spatial frequencies and age: rho = -0.44, P = 0.001 for 3 cpd; rho = -0.54, P < 0.001 for 6 cpd; rho = -0.54, P < 0.001 for 12 cpd and rho = -0.48, P < 0.001 for 18 cpd. However, no significant correlations were found between age and CSDC at 0 D, rho = -0.23, P < 0.08.
| - | Defocus Curve App Mean ± SD Median (Range) |
Conventional Tests Mean ± SD Median (Range) |
Wilcoxon Test | Passing and Bablok β0 (95% CI) β1 (95% CI) |
|
|---|---|---|---|---|---|
| Agreement A | VADC at 0 D | ETDRS chart | - | - | |
| Visual Acuity (logMAR) | -0.04 ± 0.09 -0.1 (-0.2 to 0.2) |
-0.05 ± 0.08 0 (-0.2 to 0.1) |
P = 0.51 | 0 (-0.05 to 0) | 1 (0.5, 1) |
| Agreement B | CSDC at 0 D | CSF test | - | - | - |
| Contrast Sensitivity (logCS) | 0.83 ± 0.23 0.8 (0.2 to 1.3) |
1.74 ± 0.19 a 1.7 (1.4 to 2.1) 1.81 ± 0.21b 1.8 (1.3 to 2.3) 1.47 ± 0.27 c 1.5 (1.0 to 1.9) 1.05 ± 0.33 d 1.1 (0.5 to 1.6) |
P < 0.001 P < 0.001 P < 0.001 P < 0.001 |
1.03(0.9 to 1.3)* 1.0 (0.75 to 1.3)* 0.43 (-0.2 to 0.6) -0.4 (-1.05 to 0.03) |
0.83(0.5 to 1) 1.0(0.6 to 1.2) 1.25(1.0 to 2.0) 1.67 (1.17 to 2.5)* |
| Defocus (D) | -4 | -3.5 | -3.00 | -2.50 | -2.00 | -1.50 | -1.00 | -0.50 | 0.00 | 0.50 | 1.00 |
| Visual Acuity Defocus Curve (logMAR) | |||||||||||
| Median diff. | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | -0.2 |
| Mean diff. | -0.03 | 0.08 | -0.04 | 0.07 | -0.01 | 0.06 | -0.01 | 0.05 | -0.03 | 0.1 | -0.16 |
| SR | 0.08 | 0.1 | 0.07 | 0.08 | 0.08 | 0.09 | 0.08 | 0.08 | 0.06 | 0.12 | 0.18 |
| R | 0.23 | 0.28 | 0.20 | 0.23 | 0.21 | 0.26 | 0.21 | 0.23 | 0.18 | 0.34 | 0.50 |
| % ≤ 0.1 | 76.5 | 82.4 | 85.3 | 76.5 | 82.4 | 73.5 | 85.3 | 79.4 | 94.2 | 70.6 | 41.2 |
| % ≤ 0.2 | 100 | 88.2 | 100 | 100 | 97.1 | 97.1 | 97.1 | 97.1 | 100 | 88.2 | 58.9 |
| Contrast Sensitivity Defocus Curve (logCS) | |||||||||||
| Median diff. | 0 | -0.1 | -0.1 | -0.1 | -0.1 | -0.1 | -0.1 | -0.1 | -0.1 | -0.25 | -0.05 |
| Mean diff. | -0.21 | -0.23 | -0.16 | -0.26 | -0.19 | -0.22 | -0.21 | -0.23 | -0.21 | -0.30 | -0.14 |
| SR | 0.26 | 0.29 | 0.23 | 0.3 | 0.26 | 0.29 | 0.29 | 0.27 | 0.24 | 0.34 | 0.26 |
| R | 0.71 | 0.81 | 0.64 | 0.83 | 0.71 | 0.81 | 0.81 | 0.76 | 0.67 | 0.95 | 0.72 |
| % ≤ 0.1 | 58.9 | 41.1 | 50 | 47 | 53 | 47 | 44.2 | 47 | 53 | 38.2 | 47 |
| % ≤ 0.2 | 67.6 | 52.9 | 67.6 | 58.8 | 64.7 | 64.7 | 67.6 | 61.8 | 64.7 | 47.1 | 67.7 |
3.2. Experiment 2. Repeatability Along the Defocus Curve
The repeatability along the VADC was better around the location of the highest energy efficiency of the MIOLs (far distance or 0 D defocus lens). At this location, the percentage of eyes that achieved a difference equal of less than one line of VA between the two consecutive measures was 94.2%. This percentage decreased in the near vision to 76.5% (-2.50 D) but was maintained above the 70% along all the defocus curve except for the +1.00 D location. The median differences along all the defocus curve oscillated between zero lines or one line of VA except for the +1.00 D location. The repeatability coefficient (R) was below 0.1 logMAR for all the defocus lenses except for the positive lenses. The mean total time spent for conducting VADCs was 7.98 ± 2.33 minutes, with a total of 5.65 ± 1.61 minutes dedicated to the psychophysical procedure and 2.34 ± 1.37 minutes corresponding to the time spent by the clinician replacing the defocus lenses.
The repeatability results with CSDC showed greater bias than with VADC. The percentage of subjects that obtained the same CS or less than 0.1 logCS of difference (one line) between the two consecutive measures was 53% at the point of best vision and decreased along the major part of the defocus curve. The CS was a median of 0.1 logCS higher in the second trial than in the first as shown in the median differences along almost all the defocus curve. The results of repeatability along all the defocus curve are detailed in (Table 2). The mean total time spent for conducting the CSDC was 7.81 ± 2.46 minutes, with 5.94 ± 2.06 minutes dedicated to the psychophysical procedure and 1.87 ± 0.74 minutes corresponding to the time spent by the clinician replacing the defocus lenses.
4. DISCUSSION
Defocus curve is the most useful method for evaluating the subjective range of clear vision achieved with multifocal procedures. However, one of the most important drawbacks is that it is very time-consuming, which limits its use in clinical practice. Furthermore, some considerations during the measure, such as presenting random letters [7] or the defocus lenses in a random sequence [9], should be taking into account, leading to a complex procedure for obtaining reliable results and which entails an increase of the procedure time. In this study, we introduced a test for measuring VADC and CSDC in clinical practice with an iPad.
No significant differences were found between the ETDRS and the VADC at far distance (Table 1). A median difference up to one line of VA between both tests might be expected due to the repeatability of the ETDRS chart as it has been previously reported [14]. In fact, Kędzierska et al., [23] reported in the agreement evaluation of ETDRS printed versus mobile device charts that the 59% of cases achieved the same level of visual acuity and 96% had differences of one line or less. In comparison to Kędzierska et al., [23] study, our agreement can be considered good because we achieved 45.8% of eyes with the same value of VA and 96.6% obtaining differences of ≤ 0.1 logMAR and our experiment involved two different optotypes and psychophysical procedures whereas Kędzierska et al., [23] maintained the chart and they only modified the way of presentation. Therefore, our method can be considered as interchangeable at far distance with the visual acuity measured with an ETDRS standard chart but we highlight that future studies should confirm this agreement along all the defocus curve. A post-study sample size calculation was performed to confirm whether the sample of eyes included in the current study was of adequate size using the software G*Power version 3.1 [24]. We computed the number of paired measures needed to detect a true difference of 0.05 logMAR in population means (d) with type I error probability (α) given a standard deviation (s). Specifically, for a statistical power of 80%, considering d and s changes and α of 0.05, the sample size required was 24 eyes.
CS has been considered a standard measure for assessing the performance with MIOLs as it is more sensitive to small changes in optical quality than VA [10]. However, clinical studies evaluating differences in CS between MIOLs with clinical CSF tests usually measure only the far distance and have reported no significant differences for all the spatial frequencies [25-28]. These findings reveal that the usefulness of CSF clinical tests is at least questionable for detecting small differences in optical quality among MIOLs even though these differences can be shown in optical bench [29]. Therefore, we propose the measurement of CSDC which is similar to the Through Focus Response (TFR) [16] in optical bench in order to detect these small changes in optical quality along all the defocus range that may not be detected by VADC.
As it was expected, the CS at far distance measured with the new app (optotype size corresponding to 0.3 logMAR) was significantly different from all the measured spatial frequencies of the CSF (Table 1). We computed the number of paired measures needed to detect a true difference of 0.2 logCS in population means (d) with type I error probability (α) given a standard deviation (s) for the spatial frequency of 18 cpd which was the one with higher s and therefore with the higher sample required. Specifically, for a statistical power of 80%, considering d and s changes and α of 0.05, the sample size required was 21 eyes.
It is well known that letters are not comparable to sinusoidal gratings because letters contain a broad range of spatial frequencies with a predominant frequency [30]. However, our interest was to find the spatial frequency which showed a better agreement with this letter size. Theoretically, a spatial frequency of 15 cpd has the best agreement with an optotype size of 0.3 logMAR [16]. However, we found that eyes obtained a higher CS with a sinusoidal grating of 18 cpd than the achieved with the optotype size of 0.3 logMAR. An important limitation of this analysis was the difference of background luminance between tests, whereas the CSDC test had a background luminance of 250 cd/m2, the CSF test had a luminance of 85 cd/m2. In summary, our experiment showed that the theoretical spatial frequency of 18 cpd overestimates the CS obtained with a 0.3 logMAR optotype, being the theoretical approximation of 15 cpd to 0.3 logMAR not completely correct [16].
Our results showed a good repeatability along all the VADC, with differences equal or less than one line of VA between the two consecutive measures in the 70% of eyes along all the defocus curve, except for the +1.00 D of positive defocus for which the procedure was less repeatable. Unfortunately, despite many research studies have been conducted around conventional defocus curves [5, 8, 9, 31, 32], this is the first one that measure the repeatability along all the defocus curve therefore we cannot compare our repeatability with the conventional measure. Future studies should include the repeatability along the conventional defocus curve because this information is required in order to know when differences can be attributed to a real measure or to a bias due to the procedure of measurement. Besides this limitation, the reported repeatability limit of the ETDRS at far distance in a previous study (R=0.17) [14] was very close to the obtained far distance with this new app (R=0.18).
Despite our interest of not increasing the time of the trial maintaining the psychophysical staircase with the same reversals in the VADC for CSDC, we found a poor repeatability of the procedure around three times higher than that obtained along the VADC (Table 2). It was expected a poorer repeatability of the CSDC in comparison of VADC, because CS tests are generally less repeatable than VA tests [14, 33]. The median test-retest difference in the CS along the defocus curve was -0.1 logCS, which indicates that the CS was one line higher in the second trial in comparison to the first trial probably due to a learning effect (Table 2). If we compare this finding with the VADC, we see that in half the defocus curve the median difference was 0.1 logMAR, which also indicates a probably learning effect in the VADC, but less important than in the CSDC.
The great advantage of incorporating iPad devices to the clinical practice is that there exists low differences in brightness and contrast properties between models with common displays [18, 34], and new tests can be programmed without requiring a previous calibration if the test has been designed considering the mean luminance properties of several devices [18]. In addition, new metrics can be derived in real time such as the area under the defocus curve, which so far was only possible with complex analysis difficult to implement for the clinician [32]. This new metric can help to predict the visual performance before surgery based on biometric eye characteristics as we have recently demonstrated [35].
Our VADC app accomplished the purpose for which was designed, the measure of the VADC in around 8 minutes without a considerable loss of repeatability. However, the repeatability of CSDC was significantly poorer in comparison to VADC and other CS tests [18]. There are another computerized CS tests with considerable higher repeatability than the obtained in this study such as the FrACT because of its advanced psychophysical method [36], however measurement time with this tests is such as high that hardly can be considered in clinical practice for measuring defocus curves. In fact, Bach reported 1.7 minutes for measuring VA at far distance with test-retest repeatability of 0.2 logMAR [37]. This would be 18.7 minutes for 11 defocus levels with the same repeatability than the obtained in our study at far distance (R = 0.18 logMAR). Therefore, the higher time-consuming of the FrACT does not demonstrate higher repeatability than our test in VA. On the other hand, it is required to look for the optimal equilibrium between precision/time in CSDC with our App by means of increasing the number of staircase reversals (time of the trial) for improving the repeatability without increasing significantly the time required to complete the test [15, 38, 39].
CONCLUSION
We introduced the first app for an automatic fast measure of Visual Acuity Defocus Curves (VADC) and Contrast Sensitivity Defocus Curves (CSDC). The app showed a considerably lower testing time that that required with other tests designed for testing vision at one distance, and repeated for each defocus lens for conducting a defocus curve. Despite VADC was repeatable in comparison to conventional tests, CSDC should be optimized in the future in order to obtain more repeatable results. CSDC is currently not implemented in clinical studies because testing this with conventional methods would require higher time than VADC. This new application would help to incorporate CSDC in clinical research studies. The theoretical advantages of CSDC versus VADC for detecting small changes in optical quality should be demonstrated in future studies including both metrics.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethics committe of University of Alicante, Spain.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Subjects were informed and were agreed that the data collected during the exams might be used with research purposes.
CONFLICT OF INTEREST
MR-V has designed and programmed apps that are currently distributed by the Apple Store with his own developer account. The other authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
ACKNOWLEDGEMENTS
Declared none.


