All published articles of this journal are available on ScienceDirect.
In situ Observations of Porcine Ocular Surface Cells with Handheld 2K-pixel Microscope
Abstract
Introduction:
To present our findings of the porcine ocular surface that were obtained with an ultra-compact hand-held microscope that weighs less than 500 g, we examined the corneal epithelial cells with this hand-held microscope.
Methods:
This device is equipped with an automatic focusing mechanism that enabled us to observe living cells in macro to micro magnifications with a series of operations. The focus is semi-automatically adjusted by the infrared and ultrasonic distance sensor. The instrument has a commercially-available microscope objective lens of 20x or 40x magnification and has a high-resolution 2K Complementary Metal-Oxide-Semiconductor (CMOS) camera. The theoretical spatial resolution is around 300 nm with a higher Numerical Aperture (high-NA) lenses. The widefield reflectance-based imaging system is equipped with three-color visible Light-Emitting Diodes (LEDs) for use in bright environments and an infrared LED for dark environments. Ten normal and two injured porcine corneas were examined with this hand-held microscope.
Results:
Our observations showed that the corneal and conjunctival epithelial cells could be continuously observed. The epithelial cells of the central cornea, limbus, and conjunctiva were clearly seen. The epithelial cells on the injured corneal surface were also easily and clearly observed.
Conclusion:
This hand-held microscopic imaging device allows medical health care workers such as ophthalmologists and endoscopists to obtain real-time in vivo optical biopsies without collecting tissues and cells. Our system enables us to observe single cells in the superficial layers without any fluorescein or other dyes.
1. INTRODUCTION
Confocal microscopy is widely used to examine the unstained corneal and conjunctival surfaces. However, the size of the confocal scan is very broad, and the instrument is very heavy at 120-200 kg. Therefore, these large systems are often a hindrance to clinical use. In the clinic, histological analysis of suspected tissues is the best method for evaluating the morphological normality of the ocular surface. However, the histological examination requires tissue extraction, fixation, and staining in vitro, and, finally, observations under a conventional light microscope. This requires time and the availability of histopathological laboratories.
An ultracompact light-weight imaging device with an autofocus function have been developed [1]. We have verified that it can be used for examinations of the ocular surface cells of porcine eyes.
2. MATERIALS AND METHODS
2.1. Microscope
This microscope system weighs approximately 300 g and is equipped with a 20x or a 40x objective lens and a high resolution 2k x 2k Complementary Metal-Oxide-Semiconductor (CMOS) camera. A 2K resolution is a generic term for display devices or contents having a horizontal resolution of approximately 2,000 pixels. The theoretical resolution of this device is 300 nm. This device allows real-time microscopic observations of the surface tissues at the cellular level. The widefield reflectance-based imaging system has three-color visible Light-Emitting Diodes (LEDs) for bright conditions and an infrared LED with a wavelength of 850 nm for the dark condition. Thus, this system can provide clear images of the surface of tissues and deep layer structure regardless of the ambient illumination.
This system uses an identical lens with an autofocus function with a large zoom ratio determined by a vertical position sensor. In addition, this system uses a liquid lens to change the image-formation modes behind the objective lens of microscope. Therefore, we can easily perform broad-field imaging of whole organs at high magnification to examine the cell surface and subcellular structures through the same coaxial light path by a small series of operations.
2.2. Observations
The protocol for animal studies was approved by the Institutional Review Board of the Teikyo University and the Animal Care and Use Committee of Teikyo University. Animals were cared for in accordance with the guidelines of Teikyo University for the care and use of experimental animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Ten fresh porcine eyes were obtained from a Shibaura Slaughterhouse, Tokyo Metropolitan Government (Tokyo Shibaura Organ Co., Ltd., Shinagawa, Tokyo, Japan), and used in this study. The anterior macro and micro view of the surface of the porcine eyes were examined with our hand-held microscope. Colored Images were created on the CMOS camera and transmitted and stored in a computer online. This device is equipped with a digital video camera and has no analytical tool. The captured video images can be automatically transferred from the camera to a computer with a USB cable. In 2 eyes, the corneal epithelium was scraped with a cutter, and the injured corneal surface was examined with the hand-held microscope.
3. RESULTS
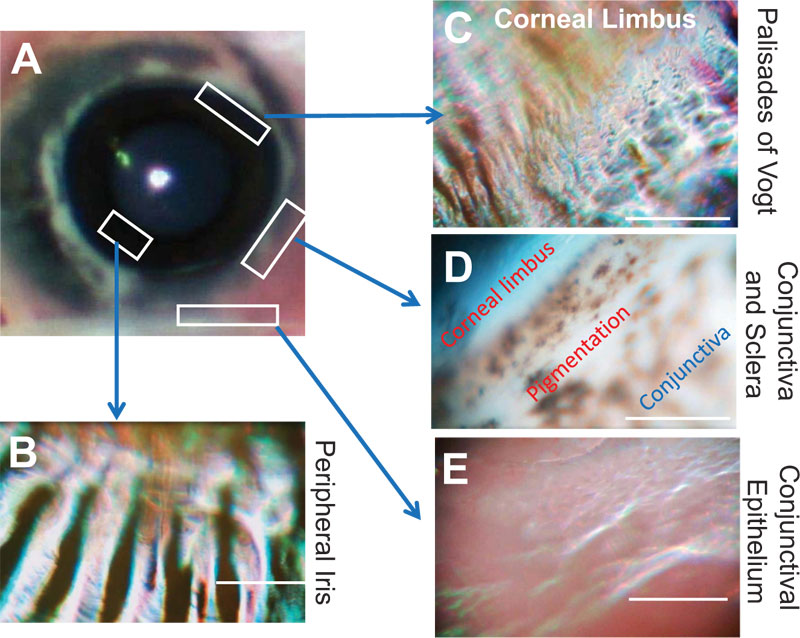
We were able to examine the porcine eyes from the macro to microscopic levels of the ocular surface through the same coaxial observational system. The surface of the cornea and conjunctiva was clearly visible in all 10 eyes (Figs. 1A and 1B). Fig. (1A) shows the entire porcine eye, and Fig. (1B) shows a zoomed view of the corneal epithelial cells. Uniform and regular corneal epithelial cells were observed in the microscope mode. Corneal limbal epithelial cells and conjunctival epithelial cells were also clearly observed because this device has an autofocus function (Fig. 2).
The corneal epithelial surface damaged by the cutter was uneven (Figs. 1C and D). Moreover, the whole ocular surface could be easily observed by a series of operations from the corneal epithelium to the corneal limbus and the conjunctival epithelium (Fig. 2).

4. DISCUSSION
Our hand-held microscope enabled us to observe cells on the ocular surface with high resolution (2K × 2K pixels). This level has approximately the same resolution as the conventional slit-lamp microscope. The nominal spatial resolution is 300 nm, which is higher than that of a conventional microscope (500 nm), and the system can be minimized to hand-held size, which weighs approximately 300g. Therefore, it can be easily adapted for clinical use even in a small examination room.
This device did not use a slit lamp biomicroscope or anterior segment OCT-confocal microscope. The basic structure is a simple structure in which an objective lens of microscope and a general CMOS digital camera are attached. This microscope consists of an inexpensive commercially available lens and camera purchased at an electronics store. Therefore, the development cost is very low.
In vivo confocal microscopy is currently used to observe ocular surface cells in specialized outpatient clinics for corneal diseases at a university hospital. However, the confocal microscope has a large system size, is not portable, and is expensive; therefore, the device is not suitable for carrying or clinical operation. Another drawback of the in vivo confocal microscopy for the cornea is that the image is black-and-white, and the working distance is very short. Our newly-developed hand-held microscope is ultracompact and light-weight, has an autofocus function and is capable of recording color images. This handy device is non-contact and non-invasive. This microscope allows us to see the histology of corneal diseases, such as corneal vascularization, trauma, infection, and corneal dystrophy that cannot be seen with a slit lamp microscope. These advantages allow non-invasive diagnosis of corneal diseases using our hand-held microscope to promise for clinical application.
Several papers described the histology of the surface of a normal human eye [2]. The corneal epithelium is composed of a uniform non-keratinized stratified squamous epithelium with 5-7 cell layers [2]. The surface layer of the conjunctiva is composed of non-keratinized stratified squamous epithelium with numerous goblet cells. Melanocytes are normally present in the basal layer of the epithelium [3]. The anterior surface of the iris is an incomplete layer of fibroblasts [4]. The anterior chamber angle is a part of the eye located between the cornea and iris, which contains the trabecular meshwork. The lens capsule is a clear, membrane-like structure. The lens capsule is a clear envelope with a membrane-like structure that surrounds the entire lens [4]. Our results showed almost the same findings as to the cell tissue image on the ocular surface of the normal human. The smoothness of the surface of the eye was easily determined from the stereoscopic images of the eyeball and also the microscopic images of the ocular surface by our hand-held microscope through a series of operations. The pathological conditions of corneal epithelial defects after corneal injury were also easily detectable by the device. Thus, the device can immediately provide real-time and in-situ images of an injured cornea in detail at the scene of an accident or when a patient cannot visit the hospital when the cornea is injured due to other factors. In addition, nursing home doctors will be able to observe patients at their sites without having to visit a hospital.
As a study limitation, we could not provide enough information and images of this device because this device was still a prototype. Additionally, this device enables us to see the tissue surface. As this device is, in principle, the same as a microscope; therefore, it is difficult to see cells in opaque tissue or deep layers. However, there are several additional advantages of this hand-held microscope over conventional microscopes. First, the imaging system does not require fluorescent dyes or contrast agents. Second, the device has a compact design and is close to clinical application. Thirdly, this device is capable of recording both the far-field and near field by an autofocus function. The cost of this microscope is in the hundreds of dollars due to the use of normal off-the-shelf lenses, sensors and cameras.
CONCLUSION
It has been concluded that our hand-held microscope allowed us to observe the ocular surface in vivo without collecting ocular tissues and cells as is done presently. We were able to examine the ocular surface of porcine eyes, and we plan to clinically observe human corneas using this imaging device.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The protocol for animal studies was approved by the Institutional Review Board of the Teikyo University and the Animal Care and Use Committee of Teikyo University, Teikyo, Japan.
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. Animals were cared for in accordance with the guidelines of Teikyo University for the care and use of experimental animals and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (16K11332).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


