All published articles of this journal are available on ScienceDirect.
Therapy of Age-related Exudative Macular Degeneration with Anti-vascular Endothelial Growth Factor Drugs: An Italian Real Life Study
Abstract
Aim:
To evaluate the real utilization of ranibizumab and aflibercept in the daily management of patients with neovascular age-related macular degeneration (nAMD) treated at the Eye Clinic of Campania University L.Vanvitelli.
Background:
Therapy with anti-vascular endothelial growth factor represents the gold standard in wet age-related macular degeneration. There are nonreal life italian studies of this therapy in the literature.
Objective:
To analyze in our sample the post-therapy variations of best-corrected visual acuity (BCVA) and central retinal thickness (CRT) observed at the end of a 12-month follow-up period.
Methods:
This real-life study analyzes 109 patients that underwent monthly checks for the first 4 months and then every 2 months until the end of the 12-month follow-up. The sample was first analyzed in its entirety, subsequently subdivided into 3 groups based on baseline BCVA, age, and the number of intravitreal injections performed, in order to identify possible predictive elements of the anti-VEGF response.
Results:
On average, patients underwent 4.16 ± 1.58 intravitreal anti-VEGF injections in 1 year. At the end of the 12-month follow-up, the patients’ average BCVA increased from 33.01 letters to 33.75 letters (+0.74 ± 9,4 letters), while the average CRT decreased from 346.86 µm to 265.39 µm (-81.47 ± 121 µm).
Conclusion:
The study shows the efficacy of anti-VEGF therapy in the stabilization of BCVA in nAMD, confirming the differences in visual outcomes compared to clinical trials, mainly for economic-organizational reasons.
1. INTRODUCTION
Age-related macular degeneration (AMD) is one of the most frequent eye diseases in Western countries, where it is still the main cause of legal blindness in the elderly population [1-3]. AMD is a degenerative disease that affects the macula and can sometimes lead to complete loss of central vision. There are two forms of AMD: a) atrophic (dry), less aggressive and with a slower evolution, and b) the neovascular type (nAMD) characterized instead by the growth of new abnormal vessels in the subretinal space with consequent accumulation of fluid and intraretinal blood that can permanently and rapidly compromise central vision [4, 5].
It has now been widely demonstrated that VEGF is the key protein that stimulates the growth of abnormal vessels. In fact, today, intravitreal injection of anti-VEGF is the standard of care for nAMD [6]. Recent studies have highlighted a reduction in blindness associated with AMD, attributing this result both to an improvement in diagnostic procedures with the consequent achievement of an early diagnosis and to the introduction of anti-VEGF [7, 8]. In daily clinical practice, the three anti-VEGFs mostly used in the management of nAMD worldwide are ranibizumab, aflibercept, and bevacizumab. Ranibizumab (Lucentis, Genentech Inc./Novartis) was approved after the phase III MARINA and ANCHOR clinical trials, which showed a security profile and an improvement of approximately 7 and 11 letters on the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 12 months and which remained almost stable at 24 months [9, 10]. Bevacizumab (Avastin, Genentech Inc./Roche) is not registered for use in AMD and is now used off-label to treat nAMD. Aflibercept (Eylea, Regeneron Pharmaceuticals, Inc.) was approved by the Food and Drug Administration (FDA) in 2011 after demonstrating its non-inferiority in terms of best-corrected visual acuity (BCVA) and safety profile compared to ranibizumab in clinical trials VIEW 1 and VIEW 2 [11-13]. Instead, pegabtanib, which is the first drug approved by the FDA, is currently no longer used for poor visual outcomes compared to other anti-VEGFs. The three main treatment regimes currently used in the management of nAMD are fixed regime according to the labelling, pro re nata (PRN), and ‘treat and extend’. The PRN, one of the most widely used regimes, provides that patients are treated exclusively when needed, that is when there are clinical-anatomical conditions considered by clinicians to cause visual deterioration. The ‘treat and extend’ regime provides that the monthly injections were offered until no signs of disease were evident clinically and on optical coherence tomography (OCT), followed by a gradual extension of the treatment interval by 2 weeks until a maximum of 12 weeks [14, 15].
Several reviews and meta-analyses have shown equal efficacy with a comparable safety profile between ranibizumab and aflibercept in the treatment of nAMD [16, 17].
However, the clinical-functional data emerging from daily clinical practice often diverge greatly from those of large randomized clinical trials (RCTs). One of the main reasons for this divergence is that the population analyzed in RCTs is highly selected and therefore does not reflect the characteristics of the real-world population. Another important factor to consider, which emerges from real-life studies, is that in daily clinical practice, patients with nAMD receive a lower number of injections or are subjected to a different treatment regime than those indicated on the label [18, 19].
The aim of this retrospective study was to evaluate the real-life utilization and visual outcomes associated with anti-VEGF therapy (ranibizumab and aflibercept) in patients with nAMD not previously treated at our Eye Clinic of Campania University “L. Vanvitelli “, Naples, during a 12-month follow-up.
2. MATERIALS AND METHODS
This is a single-center retrospective study in which patients with nAMD treated with anti-VEGF drugs (ranibizumab and aflibercept) were evaluated. The study was conducted in accordance with the Declaration of Helsinki and informed consent was obtained from all patients. The analyzed clinical sample consists of 109 patients who visited the Eye Clinic of Campania University Luigi Vanvitelli between 1 July 2017 and 1 July 2018. The patients underwent monthly checks for the first 4 months and then every 2 months until the end of the 12-month follow-up. Eye checks consisted of best-corrected visual acuity (BCVA) measurement using ETDRS charts, slit-lamp biomicroscopy and ocular fundus examination, Optical Coherence Tomography (OCT) examination with Zeiss-Cirrus OCT. Patients previously treated with anti-VEGF and photodynamic therapy (PDT) in our department and patients with concomitant macular pathologies were excluded. In all patients, a loading phase of 3 initial monthly injections was performed, followed by a PRN treatment regimen. The reprocessing criteria adopted after the loading phase were decrease in visual acuity of at least 5 ETDRS letters associated with evidence of intra and sub-retinal fluid at OCT, increase in central retinal thickness (CRT) of at least 100 µm, the appearance of new macular hemorrhage, the appearance of a new neovascular membrane (CNV) on fluorescein angiography (FAG), persistence of intra and sub-retinal fluid at least 1 month after anti-VEGF injection [14, 15].
The sample was statistically analyzed using the t-test, evaluating both the changes in visual acuity and the variations in CRT in the entire sample. The values were considered significant for p <0.05 and p <0.01.
Subsequently, the patients were subdivided into 3 groups based on baseline BCVA, age, and the number of intravitreal injections performed, in order to evaluate their features and try to identify possible predictive elements of the anti-VEGF response [20].
Two subgroups were identified based on the initial BCVA: A) patients who read a number ≥ 50 letters; B) patients who read less than 50 letters.
The sample was divided into two subgroups according to age: A) patients aged ≥ 80 years and B) patients aged < 80 years.
In the last analysis carried out, based on the number of intravitreal injections (IV), the sample was divided into the following groups: A) patients who did a number ≥ 5 IV and B) patients who did less than 5 IV.
The main purpose of this retrospective study was to evaluate the real utilization of ranibizumab and aflibercept in the daily management of patients with nAMD treated at the eye clinic of Campania University L.Vanvitelli, analyzing the variations of BCVA and CRT observed at the end of a 12-month follow-up period.
3. RESULTS AND DISCUSSION
The average age of the 109 patients analyzed was 77.14 ± 7.8 years, of which 40.9% were male (n = 45), and 59.1% were female (n = 64). (Table 1).
On average, patients underwent 4.16 ± 1.58 intravitreal anti-VEGF injections in 1 year; 54.1% (n = 59) practiced ranibizumab and the remaining 45.9% (n = 50) practiced aflibercept.
| Population n. | Mean age (SD) | Sex, n. (%) |
Mean baseline BCVA (letters) |
Mean baseline CRT (µm) |
Mean intravitreal injection |
Patients practiced Aflibercept n(%) |
Patients Practiced Ranibizumab n(%) |
|---|---|---|---|---|---|---|---|
| 109 | 77,14 ± 7.8 | Male 45 (40.9%) | 33.01 | 346.86 | 4.16 ± 1,58 | 50 (45.9%) | 59 (54.1%) |
| - | - | Female 64 (59.1%) | - | - | - | - | - |
At the end of the 12-month follow-up, the patients’ average BCVA increased from 33.01 letters to 33.75 letters (+0.74 ± 9,4 letters), while the average CRT decreased from 346.86 µm to 265.39 µm (-81.47 ± 121 µm) (Fig. 1). These data did not show a statistically significant difference either with regard to the variation of the visus (p> 0.747) or with regard to the variations of CRT (p> 3.3), but only a clinically and not statistically significant improvement in BCVA and CRT at the end of the loading phase was observed. Similarly, there were no statistically and clinically significant differences between patients treated with ranibizumab and those treated with aflibercept.
The two subgroups identified based on the number of IV were homogeneous for age, baseline BCVA and CRT; there were no significant differences between the two subgroups regarding the final BCVA and CRT (p>0,74; p>0,31). The patients who performed a number of IV ≥ 5 IV (n = 34) showed no significant differences between the initial and final visus (p> 0.9), but a significant reduction was found between the initial and final CRT (p <0.01). Instead, in the subgroup that performed less than 5 IV (n = 75), no significant differences were found neither between the initial and final visus nor between the initial and final CRT (p> 0.6 and p> 1.9) (Table 2).
| - | Mean age | Mean baseline BCVA (letters) |
Mean final BCVA(letters) |
Mean baseline CRT (µm) | Mean final CRT (µm) |
|---|---|---|---|---|---|
| Group A (n=34) | 76 | 32.7 | 33.9 (1.3) | 351.3 | 260.2 (91.1) * |
| Group B (n=75) | 77.2 | 34.9 | 35.6 (0.7) | 351.7 | 277 (74.7) * |
In the group of patients who read a number ≥ 50 letters (n = 29) at baseline, no significant differences were found between the initial and final visual acuity values (p> 0.4), but significant differences were found between the initial and final CRT (p <0, 01). However, in the group of patients with baseline BCVA less than 50 letters (n = 80), no significant differences were found, neither between the initial and final visus nor between the initial and final CRT (p> 0.3 and p> 2.8) (Table 3). There were no significant differences between the two subgroups regarding the final BCVA and final CRT.
| - | Mean age | IV number | Mean final BCVA (letters) |
Mean baseline CRT (µm) | Mean final CRT (µm) |
|---|---|---|---|---|---|
| Group A (n = 29) | 74.8 | 3.4 | 54.7 (2) | 302.9 | 238.3 (64.6) * |
| Group B (n = 80) | 78.4 3.2 | 31.4 (2.16) | 350.8 | 265.6 (85.2) |
Finally, the two subgroups identified on the basis of age were homogeneous regarding the number of intravitreal injections, initial BCVA, and initial CRT. In the subgroup of patients aged ≥80 (n = 49) years, no significant differences were found between initial and final BCVA (p> 0.98), while a significant reduction of CRT was found compared to baseline (p <0.01). Instead, in the subgroup of patients aged < 80 (n = 60) years no significant differences were found, neither between the initial and final BCVA nor between the initial and final CRT (p> 0.6 and p> 1) (Table 4). Also, in this case, there were no significant differences between the two subgroups at the end of the follow-up.
| Mean age | IV number | Mean baseline BCVA (letters) |
Mean final BCVA (letters) |
Mean baseline CRT (pm) | Mean final CRT (pm) |
|
|---|---|---|---|---|---|---|
| Group A(n=49) | 83.94 | 3.7 | 31 | 31,05 (0,5) | 330 | 260 (70) * |
| Group B(n=60) | 71.4 | 3.47 | 33.9 | 35.5 (1.6) | 368.1 | 264.4 * |
No adverse effects, such as cardiovascular events or endophthalmitis, occurred during the study.
CONCLUSION
This study represents the first Italian real-life study in the management of patients with nAMD, which demonstrated how anti-VEGF therapy, using a PRN regimen after the initial loading phase, is effective in stabilizing BCVA during a follow-up of 12 months. Both ranibizumab and aflibercept were used, showing no differences in terms of BCVA or CRT improvement, in agreement with the main RCTs and real-life studies [20-24].
Analyzing the BCVA average trend during the follow-up, an improvement is evident, with concomitant reduction of the mean CRT during the loading phase, followed by a substantial stabilization to baseline values until the end of the follow-up (Fig. 1).
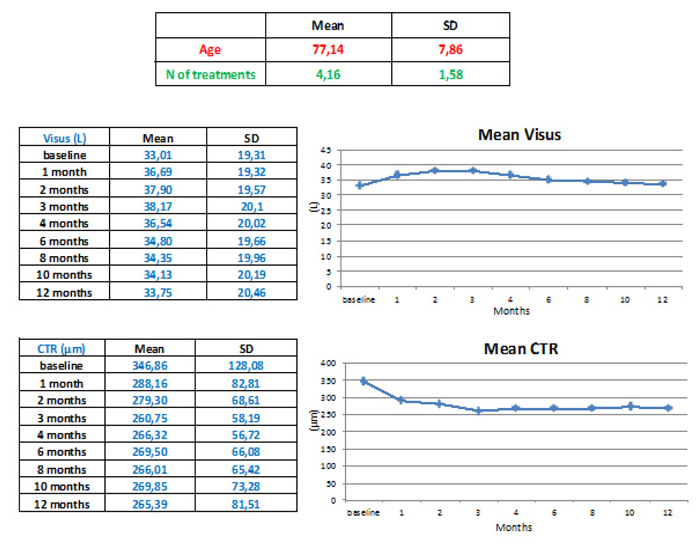
Although the average visual acuity at the end of the follow-up has slightly improved but not significantly (+0.74 ± 9,4 letters), it stands at lower levels compared to randomized clinical trials [9, 10, 25, 26] and to real-life studies [27-30]. In fact, in the global AURA study, the mean change in BCVA was +2.4 letters (5.0 injections) in year one and +0.6 letters (2.2 injections) in year two [23].
Instead, in a study on the real-life use of anti-VEGF in Germany, Ziemssen F. et al. (Ophthalmologe 2015) found an improvement of 1.1 letters to a year of follow-up, while Garweg et al. (J Ocul Pharmacol Ther. 2017) in a recent comparative retrospective analysis observed an improvement of about 4.5 letters after one year of treatment with aflibercept and about 4.2 letters after one year of treatment with ranibizumab.
The poor visual recovery observed in our study could be due to several factors: a) reduced number of injections despite a high number of monitoring visits; b) delay between the first visit and the start of treatment; c) many patients who were considered naive (since they were never treated at our eye clinic), had already undergone treatment elsewhere in previous years, and came late to our observation after reactivation of the disease. First of all, it is well known by all real-life studies that it is often very difficult to carry out high-frequency treatment schedules of injections for economic and organizational reasons. This problem is further burdened by the growing number of patients suffering from nAMD and by the unavailability found in our eye clinic to have an operating room dedicated to performing intravitreal injections in that period and with the right frequency. However, despite the fact that the average number of injections performed in our study (4.16 ± 1.58) is lower compared to that of RCTs, it appears to be in line with recent real life studies [29, 30], and in any case allowed a visual stabilization with respect to the baseline even after 12 months.
Another important factor that can explain the poor visual recovery observed in this retrospective study is the delay between the onset of the CNV and the beginning of treatment. In fact, in our experience, the waiting time between the patient's enrollment and the start of injections was, on average, about 22 ± 1,4 days. This delay, according to literature data, represents a negative prognostic factor for the possible visual improvement that can result from injection therapy [31].
Finally, another relevant factor to consider is that many patients (n = 19) came to our observation with a very advanced form of nAMD, with a very low visus (<10 letters) and with extensive retinal alterations (e.g., initial subretinal fibrosis, breaking of the IS-OS junction and of ELM, intraretinal hemorrhages) which were negative prognostic factors. Patients were often late to undergo an eye examination because they attributed their visual impairment to other problems such as cataracts and underwent control only when the contralateral eye was also affected by the nAMD.
However, our results are in agreement with AURA, which shows that the worst visual change in the first year is observed in Italy (0 letters with 3.8 rabinizumab injections), while the best one is recorded in the UK (+6 letters with 5.8 injections). Instead, our results are better than those observed by Silva R. et al. (Acta Med Port. 2017) in a recent retrospective analysis on the management of nAMD in Portugal, which describes a worsening of -1.6 letters at 12 months from the baseline with an average number of 3.8 injections of ranibizumab during the first year of treatment.
The patients who performed a number ≥ 5 IV showed no significant differences between the initial and final visus but a significant reduction was found between the initial and final CRT. Instead, in the subgroup that performed less than 5 IV, no significant differences were found, neither between the initial and final visus nor between the initial and final CRT.
In the patients who read a number ≥ 50 letters at baseline, no significant differences were found between the initial and final visual acuity values, but significant differences were found between the initial and final CRT. However, in the group of patients with baseline BCVA less than 50 letters, no significant differences were found, neither between the initial and final visus nor between the initial and final CRT.
In the subgroup of patients aged ≥ 80 years, no significant differences were found between initial and final BCVA, while a significant reduction of CRT was found compared to baseline. Instead, in the subgroup of patients aged <80 years, no significant differences were found, neither between the initial and final BCVA nor between the initial and final CRT.
Ultimately, our study confirms that the results of real-life are often different from those of RCTs, showing the efficacy of anti-VEGF therapy in visual stabilization 1 year after baseline, even using a PRN regimen after the initial loading phase, with an average number of injections lower (4.16 ± 1.58) than that of the main RCTs. Therefore, although the monitoring and therapeutic scheme proposed by main RCTs is the gold standard, according to recent studies, in order to optimize the functional results of patients with nAMD and treated with anti-VEGF, other factors should be considered beyond the number of injections such as the coexistence of systemic risk factors, the individual response and the relative sensitivity of patients to the various anti- VEGF, sudden interruption of treatment, inadequate adherence to the proposed therapeutics or monitoring scheme, the increase over time of geographic atrophy with the consequent disruption of the retinal architecture and damage of photoreceptors, as a result of either the progression of the disease or the therapy with anti-VEGF [32-35].
Evaluating the visual course in the different subgroups analyzed, we tried to identify possible predictive elements of the response to anti-VEGF therapy. While not identifying correlations and statistically significant differences between the subgroups analyzed, we observed that an important prognostic factor to consider for the purpose of visual recovery is the initial BCVA. The analysis of the subgroups divided according to the initial BCVA showed poor visual results in the group of patients with a very low initial BCVA (<10 letters), that although presenting an important improvement of the CRT, maintained the low visus in 80% of the cases and even worse in 6.7% of the cases showing instead, a slight improvement only in 13.3% of the cases. Instead, as already known, patients in this study who had a good initial BCVA (≥ 50 letters) had worse results. On average, these patients worsened by 2 letters. This was probably due to the so-called roof effect [34, 35].
Finally, the patient subgroups that at baseline had an intermediate vision, between 10 and 32 letters and between 33 and 49 letters, showed an improvement of +1.45 letters and +3.15 letters, respectively, highlighting that the presence of an intermediate vision at the time of diagnosis, represents a positive prognostic factor of response to anti-VEGF therapy.
Also, the analysis of patients on the basis of age did not reveal statistically significant differences regarding the variation of the visus, between patients aged less than 80 years and those of older age. Despite this, however, it was possible to detect that younger patients (<80 years) had a greater BCVA improvement (+1.6 letters) compared to that of older patients (≥ 80 years) (+0.05 letters); this result appears to be in agreement with various RCTs and real-life studies in which it is clear that age is a parameter that has an inverse proportionality with respect to the visual acuity gain [9-11, 27-29].
Finally, the analysis of subgroups divided by the number of intravitreal injections did not reveal statistically significant differences regarding the variation of BCVA. The disappointing result of the group of patients who had a greater number of intravitreal injections might be due to the non-optimal timing of therapy (average time between 2 injections was between 41.5 ± 2.3) and to the presence of various more advanced forms of nAMD, that required a greater number of injections, without having proportional improvements in visual acuity.
This retrospective real-life study, albeit with limitations due to a small sample and a very heterogeneous presentation of the disease compared to RCTs, demonstrates the efficacy of anti-VEGF therapy in nAMD in stabilizing BCVA, which is in agreement with the literature data. Furthermore, it is evident that in order to obtain better functional results, particular attention must be paid to the early diagnosis of neovascularization and the timing between the diagnosis and initiation of intravitreal therapy while trying to carry out a customized therapy with an adequate number of intravitreal injections.
LIS OF ABBREVIATIONS
| AMD | = Age-related Macular Degeneration |
| BCVA | = Best-Corrected Visual Acuity |
| CNV | = Neovascular Membrane |
| CRT | = Central Retinal Thickness |
| ELM | = External Limiting Membrane |
| ETDRS | = Early Treatment Diabetic Retinopathy Study |
| FDA | = Food and Drug Administration |
| IV | = Intravitreal Injection |
| nAMD | = neovascular Age-related Macular Degeneration |
| OCT | = Optical Coherence Tomography |
| PDT | = Photodynamic Therapy |
| PRN | = Pro Re Nata |
| RCTs | = Randomized Clinical Trials |
| VEGF | = Vascular Endothelial Growth Factor. |
AUTHORS’ CONTRIBUTIONS
Rossi S. and Gesualdo C. designed and wrote the paper. Tartaglione A, Scazzi G. B, D’Alessio A.C collected and analyzed the data. Melillo P. and Scazzi G. B. performed statistical analysis. Simonelli F. revised the paper.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethical committee of the University of Campania Luigi Vanvitelli and AORN dei Colli, Italy under Protocol Number 776, 17-12-2019.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was taken from all the participants when they were enrolled.
AVAILABILITY OF DATA AND MATERIALS
The data that used to support the findings of this study are available upon reasonable request from the [C.G] corresponding author.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENETS
Declared none.


