CASE REPORT
Atypical Macular Presentation in a FRAT Case and HANAC Syndrome, A Case Report
Felipe de Queiroz Tavares Ferreira1, *, Marjorie Fornazier do Nascimento de Queiroz2, André Luis Ayres da Fonseca1, Ana Cristina Lavor Holanda de Freitas1, Maurício Abujamra Nascimento1
Article Information
Identifiers and Pagination:
Year: 2021Volume: 15
First Page: 246
Last Page: 250
Publisher ID: TOOPHTJ-15-246
DOI: 10.2174/1874364102115010246
Article History:
Received Date: 27/12/2020Revision Received Date: 31/5/2021
Acceptance Date: 7/6/2021
Electronic publication date: 10/11/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose:
Report an atypical Familial Retinal Arterial Tortuosity (FRAT) case associated with Hereditary Angiopathy with Nephropathy, Aneurysm and Cramps (HANAC).
Methods:
The authors report the case of a female patient with FRAT and HANAC, and an asymmetric ocular presentation which is unusual in Familial Retinal Arterial Tortuosity patients.
Conclusion:
These findings help in better understanding this rare disease. HANAC Syndrome is not a common issue; thus, it is essential to understand it to make the right diagnosis. Careful ophthalmological examination plays a key role in this process since, in the herein reported case, it could have helped to diagnose a disease capable of affecting a patient’s health, although it was an atypical ocular impairment.
1. INTRODUCTION
Familial Retinal Arterial Tortuosity (FRAT) is a rare disease featured by increased arteriole tortuosity in the macular region. It often shows spontaneous macular hemorrhage and lack of contrast leakage in Fluorescein Angiography (FA) [1, 2]. FRAT presents dominant autosomal heritage associated with mutations in COL4A1 [3]. It is also associated with HANAC syndrome (Hereditary Angiopathy with Nephropathy, Aneurysm and Cramps), which is characterized by Familial Retinal Arterial Tortuosity (FRAT), cramps, hematuria, supraventricular extrasystoles, brain microangiopathy and aneurysms [4].
2. CASE REPORT
A thirty-five-year-old Brazilian white female patient reported sudden visual acuity loss in the Left Eye (LE) after she woke up - she referred to it as a “dark stain” that had emerged two days before, without progression, after a very stressful day. She reported that her right eyesight has not been so good since childhood.
The patient denied having systemic disease, smoking and using any medication. However, after being actively questioned, she admitted having microscopic hematuria, which had already been exhaustively investigated by nephrologists and urologists, as well as cramps all over her body since childhood and significantly strong headaches.
With respect to family history, she mentioned that her sister had an unspecified retinal disorder.
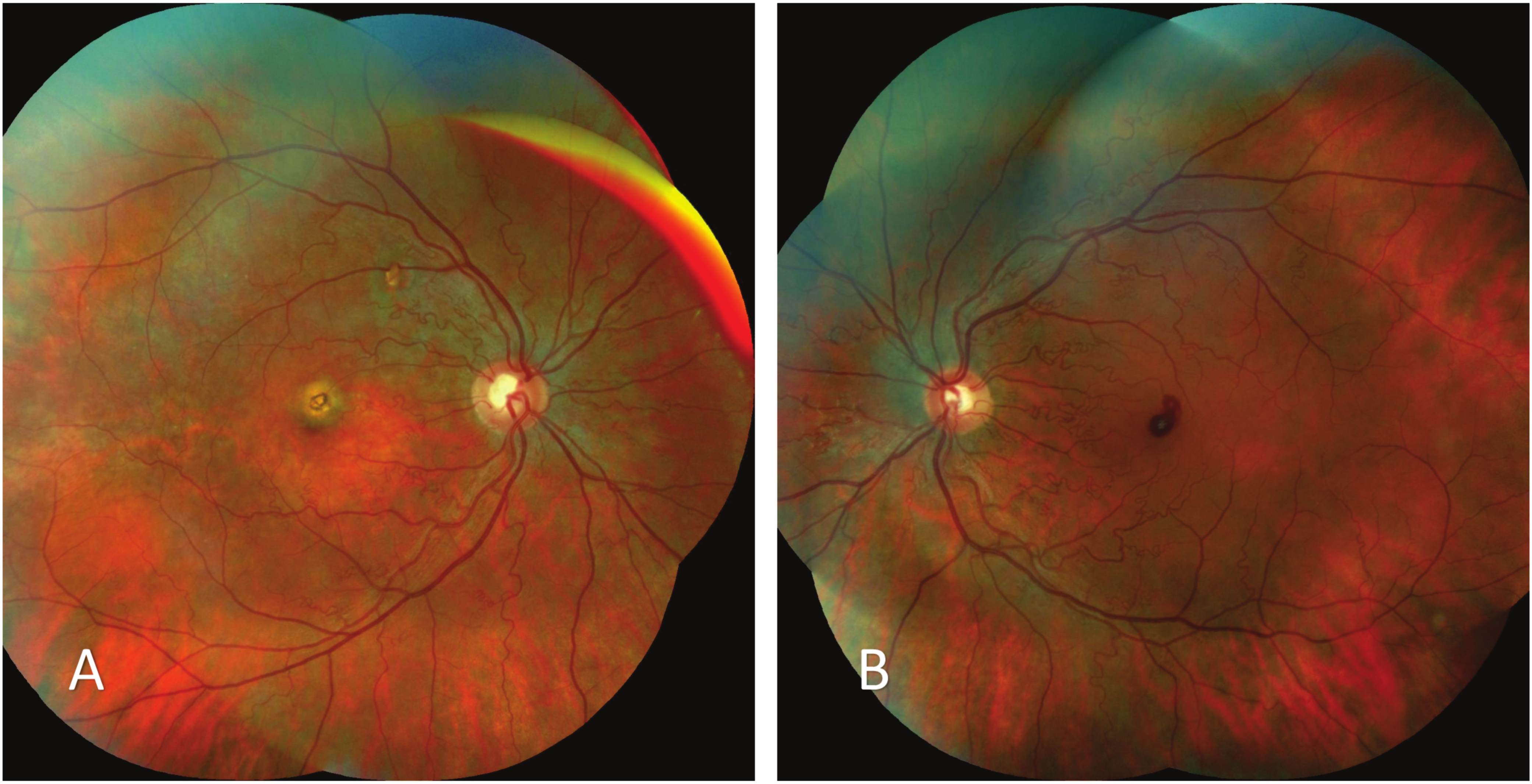
Physical examination showed Best-Corrected Visual Acuity (BCVA) of 20/25 in the Right Eye (RE) and 20/80 in LE, based on Snellen charts, as well as a spherical equivalent of +0.50 in RE and +0.25 in LE. Biomicroscopy did not show changes, however, fundoscopy showed increased arterial tortuosity in both eyes, retinal and choroidal round foveal atrophy in RE and well-defined superficial round foveal hemorrhage in LE (Fig. 1).
Fluorescein Angiography (FA) in both eyes ruled out both retinal filling leakage and delay. Increased tortuosity was only observed in arteries and arterioles, which enabled inferring that venous tortuosity remained unchanged. The patient presented irregular foveal hypofluorescence in LE and similar findings in the proximal region of the superior temporal arcade, which preserved their features during the exam and corresponded to the retinal and choroidal atrophy areas. A blocking image corresponding to the previously described hemorrhage was seen in the foveal region of LE (Fig. 2).
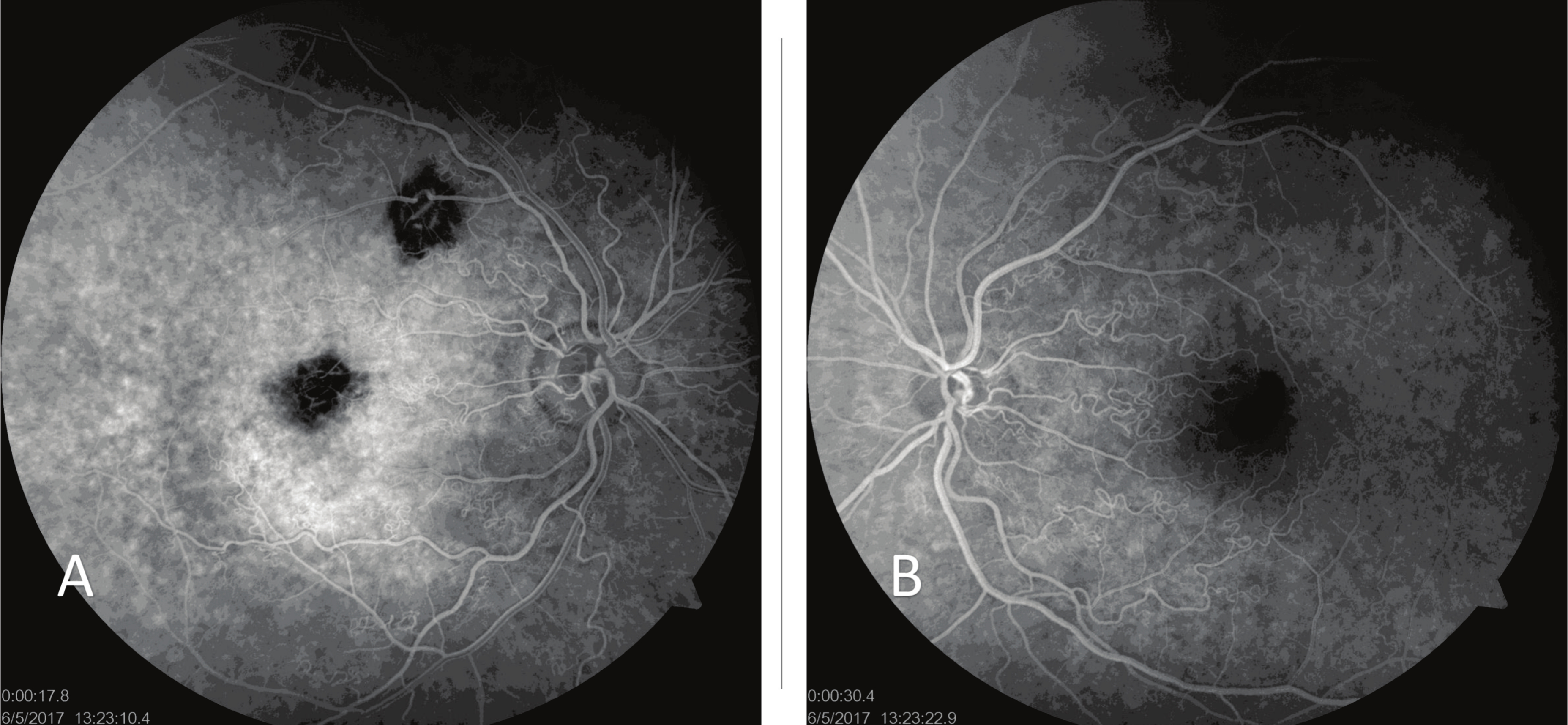 |
Fig. (2). Fluorescein angiography showing hypofluorescence attributed to atrophy in RE (A) and blockage resulting from foveal hemorrhage in LE (B). |
Indocyanine green angiography showed choroidal atrophy area in RE (Fig. 3).
Optical Coherence Tomography (OCT) showed a significant foveal depression with discontinuous outer subfoveal retinal layers and hyper adjacent choroid reflectivity, which prevailed in the foveolar region of RE. These findings were compatible with foveal retinal layers and subfoveal choroidal atrophy, likely due to a previous hemorrhage. The image of LE did not show typical foveal depression due to the accumulation of organized material in the superficial foveal region. Reflectance of this material was similar to that of retinal layers and it was attributed to superficial hemorrhage (Fig. 4). There was a difference in subfoveal choroidal thickness between both eyes (248 microns in RE and 434 microns in LE) (Fig. 5).
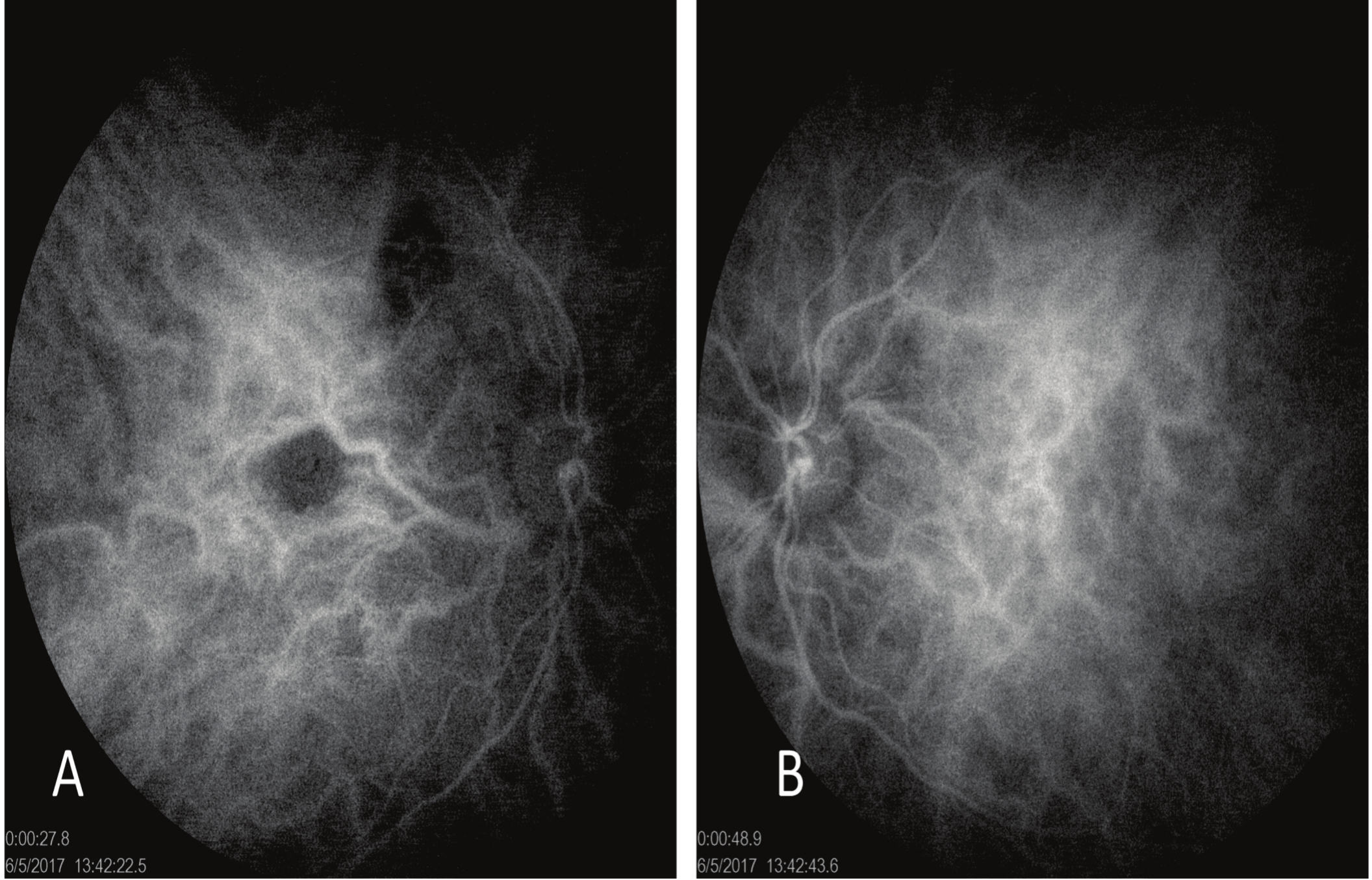 |
Fig. (3). Atrophy area in RE observed in indocyanine green angiography (A), LE did not show changes during the exam (B). |
 |
Fig. (4). OCT and Infrared findings. Retinal and choroidal atrophy in RE (A) and superficial retinal hemorrhage in LE (B). |
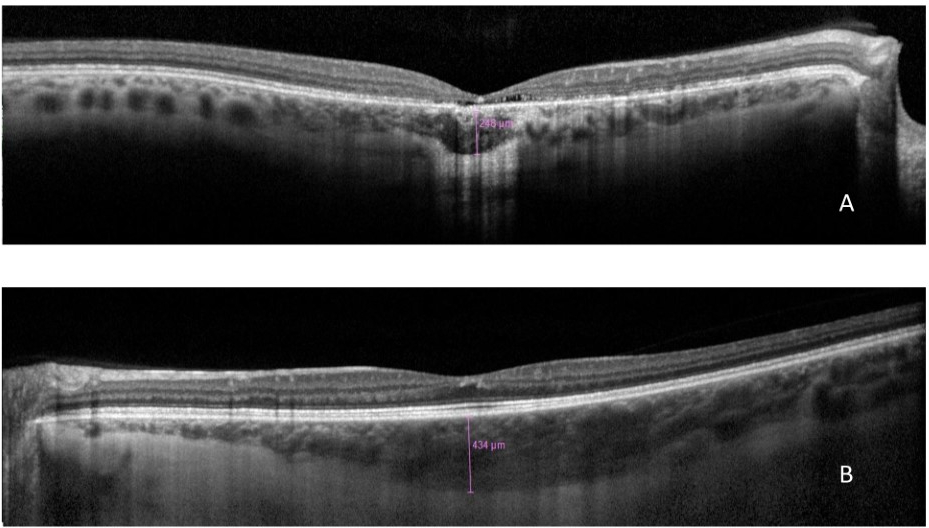 |
Fig. (5). EDI-OCT showing choroidal thickness in RE (A) and LE (B). |
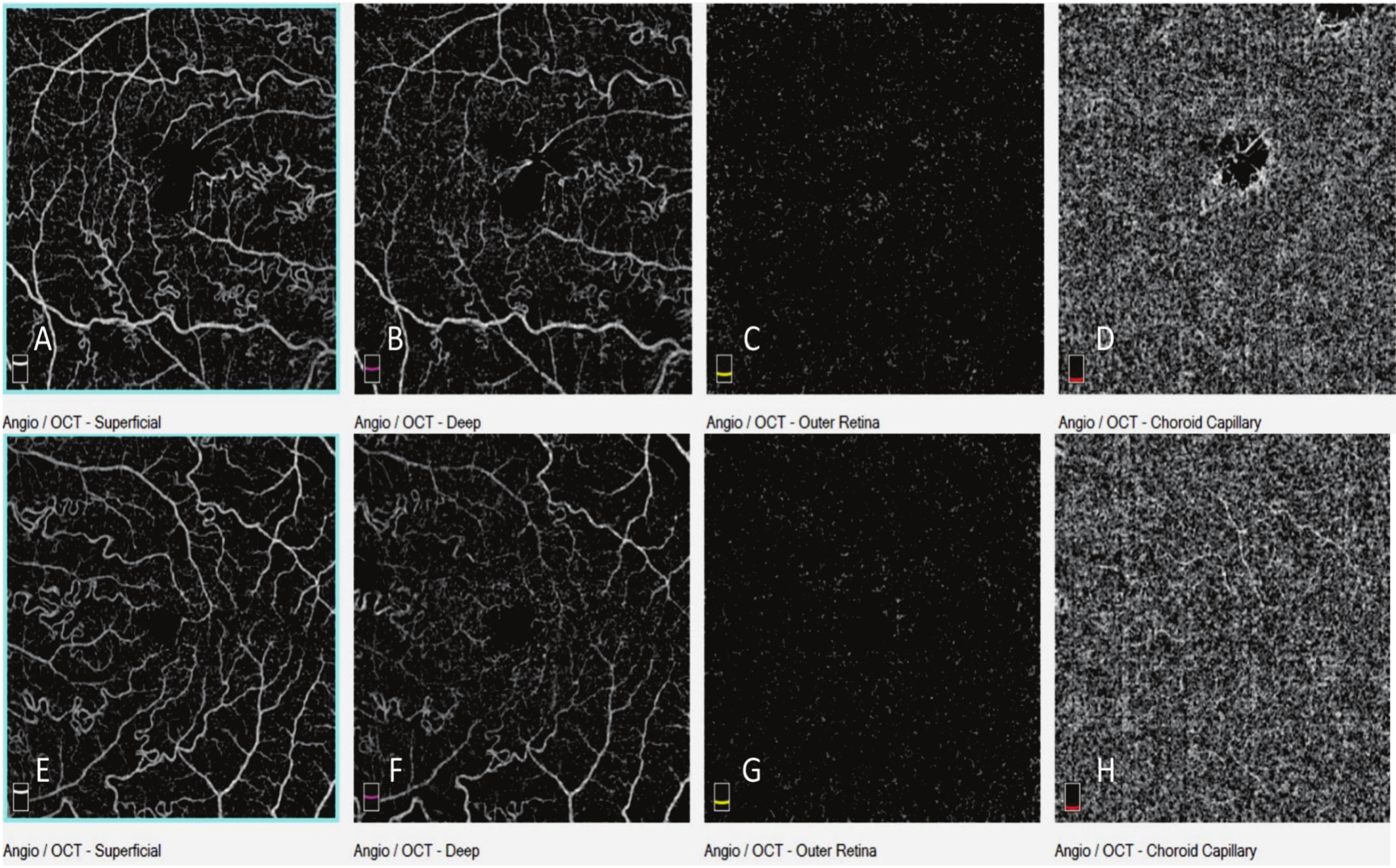
Optical Coherence Tomography Angiography (OCT-A) showed increased vascular tortuosity (Fig. 6). It also showed prevalent tortuosity in arteries and arterioles at the superficial, although not in the deep, vascular plexus, as well as deformation in the foveal avascular zone. Some artifacts can lead to deep plexus misinterpretation, however, detailed analyses enabled concluding that the plexus was not affected (Fig. 7).
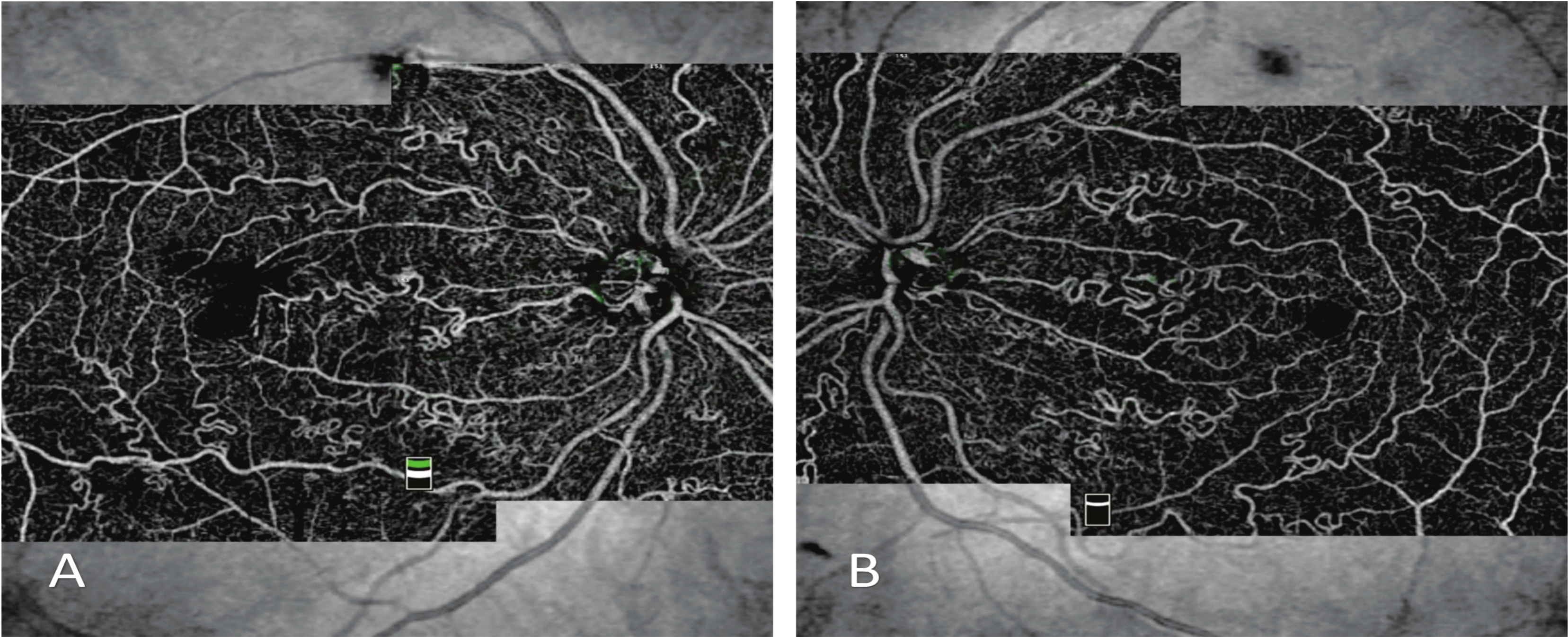 |
Fig. (6). OCT-A highlighting increased arterial and arteriolar tortuosity in RE (A) and LE (B). |
Patient’s blood pressure was lower than 120x80 mmHg in all measurements taken since the first exam - she denied having arterial hypertension.
Echodopplercardiography, basal and stress electrocardiography, hemogram, coagulogram, erythrocyte sedimentation rate, C-reactive protein, serology for acquired immune deficiency syndrome virus (human immunodeficiency virus), syphilis, hepatitis B and Mantoux tests presented normal results. Serology for Toxoplasmosis and Cytomegalovirus IgG showed positive results. Holter test showed isolated supraventricular extrasystoles and one supraventricular tachycardia episode (21 bts) - heart beating recorded 92 beats per minute. Urine tests presented hematuria, creatine kinase equal to 170 U/L (reference value for women is lower than, or equal to, 145 U/L) and glomerular filtration rate equal to 90.72 mL/min/1.72mm2. Brain tomography with contrast did not show aneurysm or changes of any sort.
The patient presented normal previous exams, including intravenous urography and electroencephalography (EEG).
2.1. Evolution
Seventeen days later, the patient was re-evaluated and reported partial eyesight improvement, although BCVA in RE and LE remained unchanged (20/25 and 20/80, respectively). Based on OCT, the superficial hemorrhage showed signs of partial reabsorption and remained as a superficial hyperreflective area in the foveal region (Fig. 8).
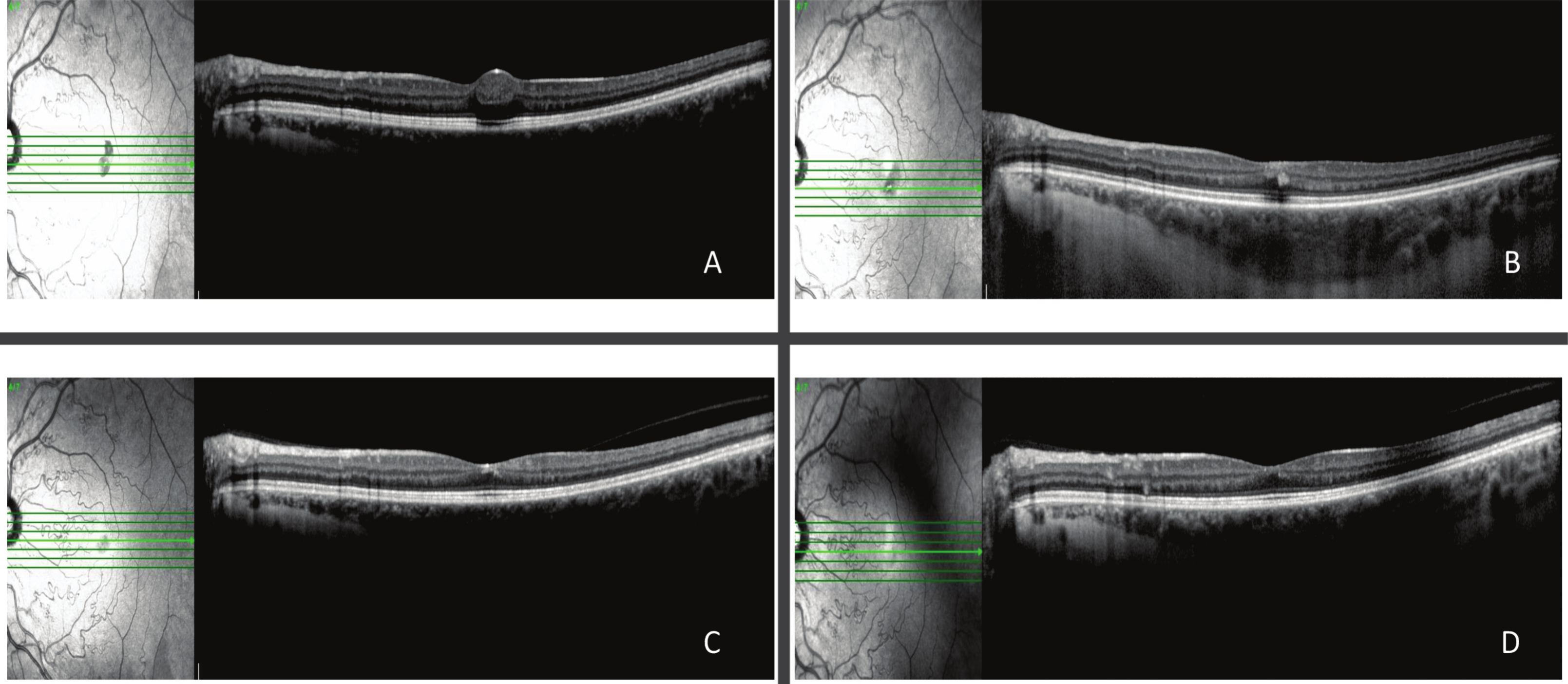 |
Fig. (8). Foveal hemorrhage reabsorption in LE, as evidenced by OCT and infrared imaging, two (A), nineteen (B), forty-nine days (C) and six months (D) after disease onset. |
Twenty days after the second evaluation, the patient returned to follow-up and reported partial eyesight improvement in LE - BCVA was 20/25 in RE and 20/40 in LE. OCT reevaluations were carried out on a monthly basis; results showed partial and progressive reabsorption of the hyperreflective material associated with foveal depression increase. Examination conducted in the last visit showed a BCVA increase to 20/20 and full hyperreflectivity reabsorption (Fig. 8). Subfoveal choroidal thickness remained unchanged in both eyes. No treatments were performed at any time, the improvement was expected.
3. DISCUSSION
Familial Retinal Arterial Tortuosity is a rare disease that was first described by Beyer in 1958 [1]. It may be associated with systemic changes such as HANAC Syndrome (Hereditary, Angiopathy with Nephropathy, Aneurysm and Cramps) [4], hippocampal infarction [5], infections, abducens nerve palsy and vascular malformations.2 The herein reported case was associated with HANAC Syndrome, cramps, increased creatine kinase level, hematuria confirmed through repeated urinalysis and supraventricular extrasystoles.
Differential diagnosis is often used to investigate diseases capable of leading to arterial tortuosity increase such as diabetic retinopathy, carotid-cavernous fistula, atherosclerotic disorders, Wyburn-Mason Syndrome, sickle-cell anemia [2], Fabry disease [6], among others [2]. FRAT may happen without systemic changes, despite the same mutation in the COL4A1 gene [3]. However, it is essential to be attentive to systemic disorders, mainly to the likelihood of aneurism development. Clinical history analysis and examination of patients’ family are recommended. Fabry disease is an important differential diagnosis, mainly because it is an inherited disease presenting significantly similar tortuosity in retinal vessels. It may cause pain episodes, kidney damage, arrhythmias and stroke, as well as present relevant skin, gastrointestinal and auditory symptoms [6]. Cornea verticillata is an important ophthalmologic sign of Fabry disease, which can be used to make a differential diagnosis [6].
The present study reported the first asymmetric and unusual presentation of FRAT with HANAC. HANAC Syndrome is a rare disease that has been described in at least six families worldwide. It is worth emphasizing that the herein reported case was diagnosed through ophthalmological examination. The aforementioned diagnosis process was based on fundoscopy, which showed increased arterial tortuosity in both eyes, as well as on spontaneous foveal hemorrhage. After FRAT was diagnosed, the patient was examined and diagnosed with HANAC syndrome based on symptoms such as hematuria, cramps, increased creatine kinase level and isolated supraventricular extrasystoles. A genetic test was made available, but the patient did not agree to be tested. Her family lives far away from her, in a distant city, and faces social limitations that stop it from being examined.
FRAT is often asymptomatic, although it may present spontaneous retinal hemorrhages after mild trauma, physical exercises or other activities capable of increasing central venous pressure. Retinal hemorrhages can mainly take place in patients’ macula, although it can also happen in different areas, in some cases. The retinal hemorrhages tend to disappear spontaneously, without the need for treatment, similar to the case [2]. The patient in the present case attributed the visual acuity loss episode to a very stressful day; however, whether she was right or wrong about it is an assumption that remains unclear.
It is also essential to emphasize the unusual asymmetric ocular impairment presented by her. The case evolved to an atrophic lesion and compromised all foveal retinal layers, as well as to choroid in RE and to late hemorrhage in LE, which only compromised superficial retinal layers and presented progressive reabsorption. The hypothesis of the current study was that the two chorioretinal scarring areas resulted from previous retinal hemorrhages that may have happened throughout retinal development, during the patient’s childhood or fetal stage, a fact that could explain the atrophy observed in all retinal and choroidal layers. The patient reported right eyesight decrease since childhood, but she was not able to associate it with any previous episode. Hemorrhages often happen in the fovea, however, they are reabsorbed without leaving sequelae. Atrophies are somewhat similar to focal choroidal excavations [7], but the patient reported that her right eyesight remained unchanged. Her lesions remained stable during the follow-up. Retinal atrophy was already described in two mice with COL4A1 mutations [8], but not in humans.
OCT-A has shown that the disease was limited to, and only affected, the superficial plexus without compromising the deep vascular plexus; this outcome was consistent with the literature [9]. OCT-A showed increased vessel tortuosity and more details than FA, and it enabled inferring that this technique can be an important tool in FRAT diagnosis [10] Some artifacts in the exam can be confusing and make health professionals believe that the deep plexus was affected, however, careful analysis enabled seeing that it was not affected.
OCT-based evaluation showed asymmetric choroidal thickness, atrophy in RE and choroidal thickening in LE, which remained stable throughout the hemorrhage reabsorption process. It was not possible to explain why patient’s choroidal thickness was so asymmetric since it presented a difference of 186 µm between eyes.
CONCLUSION
In conclusion, although the herein reported case referred to an atypical ocular impairment type, it is essential to perform a careful ophthalmological examination, since it can help to diagnose diseases capable of affecting a patient’s health and of leading to severe systemic consequences. Thus, it is essential to understand patients’ ophthalmological presentation in order to make the right diagnosis and prevent severe consequences. Although HANAC Syndrome is not a common condition, its ophthalmological findings can be easily observed in a non-invasive way.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed consent was obtained from the participants.
STANDARDS OF REPORTING
CARE guidelines and methodologies were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the finding of the article is available in the Zenodo Repository at zenodo.org, with reference number https://doi.org/10.5281/zenodo.5543764
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.