All published articles of this journal are available on ScienceDirect.
Early Postoperative Effect of Ripasudil Hydrochloride After Trabeculectomy on Secondary Glaucoma: A Randomized Controlled Trial
Abstract
Purpose:
To evaluate the effect of Rho-associated kinase inhibitor (ripasudil hydrochloride hydrate; ripasudil) eye drops on postoperative intraocular pressure (IOP) after trabeculectomy in eyes with uveitic glaucoma.
Design:
This was a prospective, observational, controlled, and randomized study.
Methods:
Sixteen eyes of 16 patients with uveitic glaucoma who underwent trabeculectomy without mitomycin C were randomly treated without ripasudil (8 eyes) and with ripasudil (8 eyes). Postoperative IOP and surgical outcomes 3 months after surgery were compared between the two groups.
Results:
No patient discontinued treatment due to the lack of efficacy or adverse effects of ripasudil during the 3-month study period in the ripasudil group. The mean IOP (mmHg) in the control and ripasudil groups were 42.5 ± 9.8 mmHg /43.9 ± 11.7 mmHg (p = 0.82) at baseline, 14.3 ± 4.9 mmHg /9.0 ± 3.7 mmHg (p = 0.04) at 1 week, 16.3 ± 4.2 mmHg /10.6 ± 3.0 mmHg (p = 0.01) at 1 month, and 16.0 ± 3.4 mmHg /12.5 ± 2.3 mmHg (p = 0.04) at 3 months. The number of laser suture lysis procedures (2.0 ± 0.5 vs 0.4 ± 0.7), the rate of bleb revision by needling (50.0% vs 0.0%), and the mean number of antiglaucoma medications (1.6 ± 1.5 vs. 0.1 ± 0.3) after trabeculectomy were higher in the control group than in the ripasudil group (all p < 0.05). A multivariate analysis showed that the IOP reduction rate at 3 months after surgery was associated with the use of ripasudil and baseline IOP (all p < 0.05).
Conclusion:
This study demonstrated the therapeutic efficacy, safety, and tolerability of ripasudil for 3 months postoperatively. Ripasudil may effectively reduce postoperative IOP and increase the success rate of trabeculectomy in patients with uveitic glaucoma.
1. INTRODUCTION
Secondary glaucoma is a difficult and common complication of uveitis, occurring in approximately 20% of patients following uveitis [1, 2]. Intraocular pressure (IOP) elevation associated with uveitis is usually caused by increased resistance to outflow in the trabecular meshwork, which is caused by the inflammatory disruption of the blood-aqueous barrier [3-6]. Trabeculitis, inflammatory cytokines, and pigments produced by uveitis increase aqueous viscosity and protein levels, resulting in IOP elevation [7, 8]. Additionally, the use of corticosteroids to treat uveitis often leads to elevated IOP [4, 9]. Traditional trabeculectomy is the most common glaucoma surgery [10-19]. Other surgical interventions for uveitic glaucoma include trabeculectomy-modifying EX-PRESS implants [20], aqueous drainage devices (Ahmed®) [18, 21], Baerveldt® [22, 23], and Molteno® [24]. A comparative study of trabeculectomy with mitomycin C (MMC) and Ahmed valve implantation in uveitic glaucoma showed that mean IOP was reduced from 29.2 to 18.4 mmHg in the trabeculectomy group and from 33.4 to 15.5 mmHg in the Ahmed valve group [25]. Percent reduction from preoperative IOP was 31.3% in the trabeculectomy group and 42.7% in the Ahmed valve group [25]. The authors concluded that both glaucoma surgeries effectively reduce and control postoperative IOP in uveitis glaucoma over 3 years [25]. In contrast, a retrospective study showed that the 3-year success rate of trabeculectomy with MMC was 71.3% in eyes with uveitic glaucoma and 89.7% in eyes with primary open-angle glaucoma [16]. Another study showed that the persistence rates of postoperative IOP were 76.1% at ≤21 mmHg, 71.5% at ≤18 mmHg, and 68.1% at ≤15 mmHg at 5 years after surgery, and the persistence of filtered bleb was 54.4% [17]. These results suggest that trabeculectomy with MMC does not always provide good postoperative outcomes for uveitic glaucoma and is often challenging because of various uveitis-related factors. The first speculated reason may be that inflammation caused by uveitic glaucoma increases resistance to aqueous outflow after glaucoma surgery. Second, postoperative uveitic inflammation may increase adhesions at the site of the excised trabecular meshwork, scleral flaps, and subconjunctival scarring under the bleb conjunctiva [26]. Therefore, in uveitic glaucoma, regulating the aqueous outflow through bypass by trabecular meshwork excision is important.
Recently, it has been reported that a Rho-associated protein kinase (ROCK) inhibitor reduced transforming growth factor-β (TGF-β)-induced myofibroblast transdifferentiation and inhibited fibroblast growth [27]. ROCK inhibitors also prevent the differentiation of human conjunctival fibroblasts into myofibroblasts [28] and improve surgical outcomes after glaucoma filtering surgery in rabbits [29]. ROCK inhibitors to suppress postoperative fibroblast proliferation have recently come into clinical use in glaucoma surgery.
A new ophthalmic solution of ROCK inhibitors (ripasudil hydrochloride hydrate: Glanatec® ophthalmic solution 0.4%; Kowa Company, Ltd., Nagoya, Japan) was released in December 2014 in Japan. Ripasudil is usually used as a second-line treatment for glaucoma as a combination therapy and occasionally as a first-line treatment as monotherapy [30, 31]. There are two main aqueous humor outflow pathways: the trabecular outflow pathway (conventional outflow) and the uveoscleral outflow pathway (unconventional outflow) [32]. Ripasudil achieves its IOP-lowering effects by directly increasing the major aqueous outflow through the trabecular meshwork route [33]. Ripasudil has been shown to reduce IOP, even in uveitic glaucoma [34, 35]. Based on these results, ripasudil is expected to reduce IOP after trabeculectomy in uveitic glaucoma. Unfortunately, few studies have examined the effect of ripasudil on the surgical outcomes of trabeculectomy in uveitic glaucoma. Accordingly, the present study aimed to examine ripasudil's postoperative efficacy for IOP reduction and surgical outcomes after trabeculectomy in patients with uveitic glaucoma.
2. MATERIALS AND METHODS
2.1. Study Design
This was a prospective, randomized controlled study. The study was conducted at the Teikyo University School of Medicine and Tokyo Women’s Medical University Medical Center East Hospital. The study was performed following the Declaration of Helsinki. Our Institutional Review Board approved this study (#Teirin 18-203). The study was registered as UMIN000044345 by the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) in Japan. This study received no financial support from any pharmaceutical company, financial grant, or sponsorship.
2.2. Participants
The purpose and nature of the study were explained in detail, and informed consent was obtained from all patients. The first eyes of consecutive patients who underwent trabeculectomy to manage uveitic glaucoma between January 2015 and December 2020 were prospectively enrolled.
Inclusion criteria were as follows [1]: patients aged 18 years or older and [2] patients with uncontrolled uveitic glaucoma with IOP > 21 mmHg despite maximal topical antiglaucoma medications in at least one eye. The exclusion criteria were as follows: patients with [1] closed or barely open anterior chamber angle [2]; a history of acute angle-closure or ocular trauma [3]; neovascular glaucoma [4]; a history of ocular surgery including refractive surgery, glaucoma filtering surgery, or vitreous surgery [5]; a history of cataract surgery via a transconjunctival sclerocorneal incision; and [6] inability to adhere to the treatment and follow-up plan. We examined the anterior chamber angle in all patients before glaucoma surgery. Patients with a history of clear corneal cataract surgery were excluded from the study. Additionally, patients who underwent combined cataract surgery with trabeculectomy for uveitic glaucoma were excluded from this study.
The study population consisted of 16 patients with uveitic glaucoma aged 69.3 ± 9.9 years (mean ± standard deviation (SD)) with an age range of 55 to 86 years (Table 1). All the patients were Japanese residents of Japan. As for prior antiglaucoma drugs, one patient was using prostaglandin analog (PGA) + carbonic anhydrase inhibitor (CAI)+α2-agonist (AA) (6.3%), one was using PGA/beta-blocker (BB) combination + α1-blocker (AB) (6.3%), six were using PGA + BB/CAI combination (37.5%), six were using PGA + BB/CAI combination + AA (37.5%), one was using PGA + BB/CAI combination + AB (6.3%), and one was using PGA + BB/CAI combination+AA+AB (Table 1). If both eyes fulfilled the eligibility criteria, the first eye that underwent trabeculectomy was used for the analysis.
| - |
Control group [range] |
Ripasudil group [range] |
P value |
| Number of participants | 8 | 8 | - |
| Female/male | 4/4 | 3/5 | 0.500 |
| Age (years) | 68.6 ± 10.3 [55-86] |
69.9 ± 9.4 [55-85] |
0.815 |
| Duration of glaucoma at the time of the study (months) |
45.6 ± 23.5 [9-70] |
43.3 ± 26.3 [21-108] |
0.861 |
| Baseline IOP (mmHg) | 42.5 ± 9.8 [28-58] |
43.9 ± 11.7 [29-64] |
0.815 |
| Phakia / pseudophakia (IOL) | 5/3 | 5/3 | |
| Prior glaucoma medication(s) | |||
| The number of antiglaucoma eye drops | 3.4 ± 0.5 [3-4] |
3.8 ± 0.7 [3-5] |
0.248 |
| Oral decarboxylase inhibitor | 5 | 7 | 0.285 |
| PGA + CAI + AA | 0 | 1 | - |
| PGA/BB combination + AB | 0 | 1 | - |
| PGA + BB/CAI combination | 5 | 1 | - |
| PGA + BB/CAI combination + AA | 2 | 4 | - |
| PGA + BB/CAI combination + AB | 1 | 0 | - |
| PGA + BB/CAI combination + AA + AB | 0 | 1 | - |
| - | Control group | Ripasudil group |
| - | Number (%) | Number (%) |
| Anatomic classification | ||
| Anterior uveitis | 5 (62.5%) | 4 (50.0%) |
| Intermediate uveitis | 1 (12.5%) | 1 (12.5%) |
| Posterior uveitis | 0 (0.0%) | 1 (12.5%) |
| Panuveitis | 2 (25.0%) | 2 (25.0%) |
| Causes of uveitis | ||
| Herpes | 3 (37.5%) | 2 (25.0%) |
| Anterior idiopathic | 2 (25.0%) | 2 (25.0%) |
| Behçet's disease | 1 (12.5%) | 1 (12.5%) |
| Sarcoidosis | 1 (12.5%) | 1 (12.5%) |
| Unknown etiology | 1 (12.5%) | 2 (25.0%) |
2.3. Diagnosis of Uveitis and Uveitic Glaucoma
Uveitis was diagnosed and classified according to the International Uveitis Study Group criteria [36, 37]. The anatomic location of the uveitic inflammation was classified as anterior, intermediate, posterior, or panuveitis [36, 37]. Anterior uveitis was defined as primary inflammation in the anterior chamber, such as iritis and/or iridocyclitis. Intermediate uveitis was defined as inflammation primarily in the middle portion of the eye (anterior vitreous, posterior ciliary body, ora serrata, and pars plana). Posterior uveitis was defined as intraocular inflammation primarily involving the retina, choroid, retinal vessels, and/or posterior vitreous humor. Panuveitis (diffuse uveitis) is inflammation in the anterior chamber, vitreous, and retina or choroid [36, 37].
Uveitic glaucoma was defined as eyes with active or a history of uveitis and glaucomatous optic neuropathy with high IOP > 21 mmHg after the onset of uveitis [18, 21, 38].
2.4. Indication for Glaucoma Surgery
The indications for glaucoma surgery are as follows: high IOP > 21 mmHg despite maximal topical glaucoma medication, progression of visual field defect, and intolerance to topical or oral glaucoma medication [21]. All cases underwent surgery during the first attack of elevated IOP in uveitis glaucoma. All patients underwent surgery within two months after glaucoma eye drops failed to reduce IOP.
2.5. Surgical Procedure
Trabeculectomy was performed using a fornix-base technique. All surgeries were performed by the same surgeon (T.M.) after topical anesthesia with 4% lidocaine hydrochloride (Xylocaine®, AstraZeneca, Osaka, Japan), and a fornix-based conjunctival opening was created. A half-thickness of 4 × 4 mm scleral flap was created in the superior sclera. Sclerectomy, sinusotomy, and iridectomy were performed using scleral flaps. The scleral flap was secured using four 10-0 nylon sutures. The conjunctiva was closed using 10-0 nylon sutures. The Ministry of Health did not permit the use of MMC, Labour and Welfare in Japan during the study period.
2.6. Postoperative Management
Postoperative management included the topical application of antibiotic eye drops of moxifloxacin ophthalmic solution 0.5% (Vegamox® 0.5% ophthalmic solution, Novartis Pharma K.K., Minato-ku, Tokyo, Japan) four times a day for 2 weeks and two times a day over the next 2 weeks and betamethasone sodium phosphate 0.1% (Rinderon Ophthalmic, Otic and Nasal Solution® 0.1%, Shionogi & Co., Ltd., Chuo-ku, Osaka, Japan) four times a day for 2 weeks, which was tapered to two times a day over the next 2 weeks and then switched to fluorometholone ophthalmic solution 0.1% (Flumetholone eyedrops® 0.1%, Santen Pharmaceutical Co, Osaka, Japan) twice daily for a further 2 months. Follow-up visits were held on days 1–3 post-surgery, weekly until 1 month, and then every 2 weeks until 3 months after the surgery.
2.7. Laser Suture Lysis (LSL) and Needling
Selective argon LSL was performed with a Hoskins LSL lens between 4 days and 4 weeks after surgery. The indication for suture lysis was poor filtration of aqueous humor associated with tight wound closure or uncontrolled IOP (18 mmHg or more) despite digital ocular massage. The argon laser settings were 1–2 shots of 50-ɥm spot size, a duration of 0.1 s, and a power setting between 300 and 400 mW.
Needling was performed between 3 weeks and 3 months after surgery if the bleb conjunctiva adhered to the sclera. The indication for needling was non-filtering or failing blebs, even after LSL. All needling was performed with a 27-gauge needle from a single site at the slit lamp to revive flat or encapsulated blebs. A 27-gauge needle was passed into the subconjunctival space and moved several times under the conjunctiva and scleral flap to disrupt the adhered tissues. Needling continued until the revived blebs became further diffuse and elevated.
2.8. Addition of Antiglaucoma Medications
Anti-glaucoma eye drops were added to control the postoperative IOP between 4 weeks and 3 months after surgery. The indication for the postoperative addition of antiglaucoma medications was uncontrolled IOP (18 mmHg or more) even with a functioning cystic bleb or after needling and LSL. Anti-glaucoma eye drops were added in the following order until IOP dropped below 18 mmHg. It was added in the order of 0.5% timolol maleate (Timoptol ophthalmic solution® 0.5%, Santen Pharmaceutical Co), 0.5% dorzolamide hydrochloride (Trusopt ophthalmic solution® 0.5%, Santen Pharmaceutical Co), 0.1% brimonidine tartrate (Aiphagan ophthalmic solution ® 0.1%, Senju Pharmaceutical Co,. Ltd, Osaka, Japan), and 0.03% bimatoprost (Lumigan®, Senju Pharmaceutical Co,. Ltd).
2.9. Intervention
All participants were randomly assigned to two groups using a table of random numbers immediately after the surgery: the control group without ripasudil hydrochloride eye drops and the ripasudil group with ripasudil hydrochloride eye drops after the surgery.
Consequently, the study population consisted of eight control participants aged 68.6 ± 10.3 years and eight patients with ripasudil hydrochloride eye drops aged 69.9 ± 9.4 years (mean ± SD) (Table 1). There were no significant differences in age, sex, or other parameters between the two groups (Table 1). In the ripasudil group, ripasudil hydrochloride hydrate ophthalmic solution was started on the morning after surgery and administered twice daily for 3 months after surgery.
2.10. Outcomes
The age, sex, medical history, current antiglaucoma drugs, and ocular history of each patient were assessed at the baseline visit. The main outcome measures were preoperative IOP and postoperative IOP at 1 week and at 1, 2, and 3 months. At each visit, the IOP was measured using Goldmann applanation tonometry in the morning (between 9 a.m. and 12 noon) by the same ophthalmologist (TM). The percent reduction in IOP was calculated as follows: (preoperative IOP – postoperative IOP)/preoperative IOP) × 100.
The secondary outcome measures were postoperative complications and the number of postoperative antiglaucoma medications used, postoperative argon LSL of fixed scleral flap sutures, and needling of the bleb 3 months after surgery. All data were stored in a computer database during each visit.
2.11. Statistical Analysis
Continuous variables were compared between the two groups using the two-tailed paired or unpaired Student’s t-test. Frequencies were compared using chi-square analysis or Fisher’s exact test, while the Mann–Whitney U test was used to compare semiquantitative measurement variables between the two groups. Factors associated with the IOP reduction rate at 3 months after the surgery were investigated using multivariate logistic regression analysis, with the explanatory variables including the use of ripasudil after the surgery (control group = 0/ripasudil group = 1), duration of glaucoma at the time of the study (months), baseline IOP, number of antiglaucoma eyes drops before surgery, and presence of IOL (phakia = 0/pseudophakia = 1). The level of significance was set at p < 0.05. Statistical analyses were performed using SAS System software version 9.1 (SAS Institute Inc., Cary, North Carolina, USA).
3. RESULTS
The demographic features of the participants are summarized in Table 1. None of the patients discontinued the study due to the lack of efficacy or adverse effects of ripasudil during the 3 months after surgery. Transient hyphema occurred in two cases in the control group and one case in the ripasudil group and was subsequently absorbed within 1 week postoperatively in all cases. There were no complications, such as choroidal detachment, retinal detachment, or hypotony maculopathy in either the control or ripasudil groups after trabeculectomy.
The mean IOP was 42.5 ± 9.8 (range 28-58) mmHg at baseline, 14.3 ± 4.8 (range 6-22) mmHg at 1 week, 16.3 ± 4.2 (range 9-22) mmHg at 4 weeks, 16.5 ± 4.4 (range 11-25) mmHg at 2 months, and 16.0 ± 3.4 (range 13-24) mmHg at 3 months after surgery in the control group (Fig. 1). The mean IOP was 43.9 ±11.7 (range 29-64) mmHg at baseline, 9.0 ± 3.7 (range 4-14) mmHg at 1 week, 10.6 ± 3.0 (range 6-14) mmHg at 4 weeks, 12.1 ± 2.4 (range 9-17) mmHg at 2 months, and 12.5 ± 2.3 (range 9-16) mmHg at 3 months after surgery in the ripasudil group (Fig. 1). There was a significant decrease in IOP compared with baseline at all visits after surgery in both groups (p < 0.001, paired two-tailed Student's t-test) (Fig. 1). There was also a significant difference in IOP between the control and ripasudil groups at all time points after the surgery (p < 0.050, unpaired two-tailed Student's t-test) (Fig. 1).
The mean percent decrease in IOP (IOP reduction rate) was larger in the ripasudil group than in the control group at 1 week (80.0 ± 5.4% vs. 64.9 ± 15.1%, p = 0.036), 4 weeks (75.3 ± 6.4% vs. 60.3 ± 13.0%, p = 0.020), 2 months (71.0 ± 7.0% vs. 60.2 ± 9.7%, p = 0.033), and at 3 months after surgery (70.3 ± 6.6% vs. 61.3 ± 6.9%, p = 0.027) (unpaired two-tailed Student's t-test) (Fig. 2).

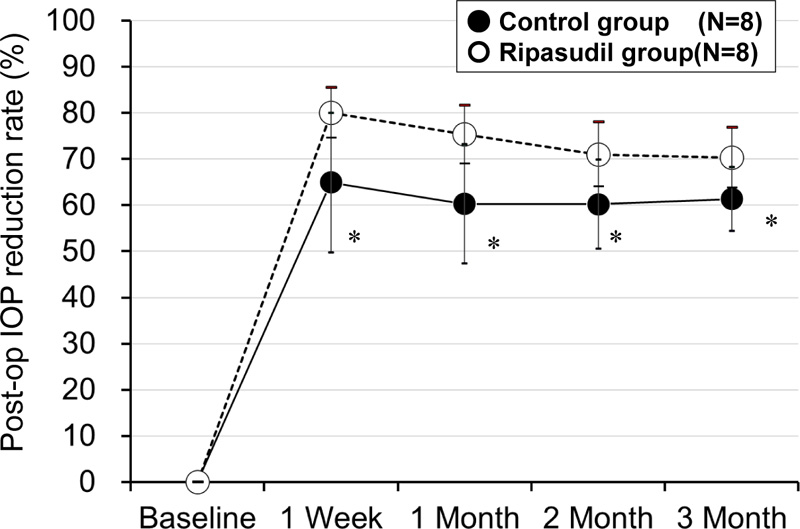
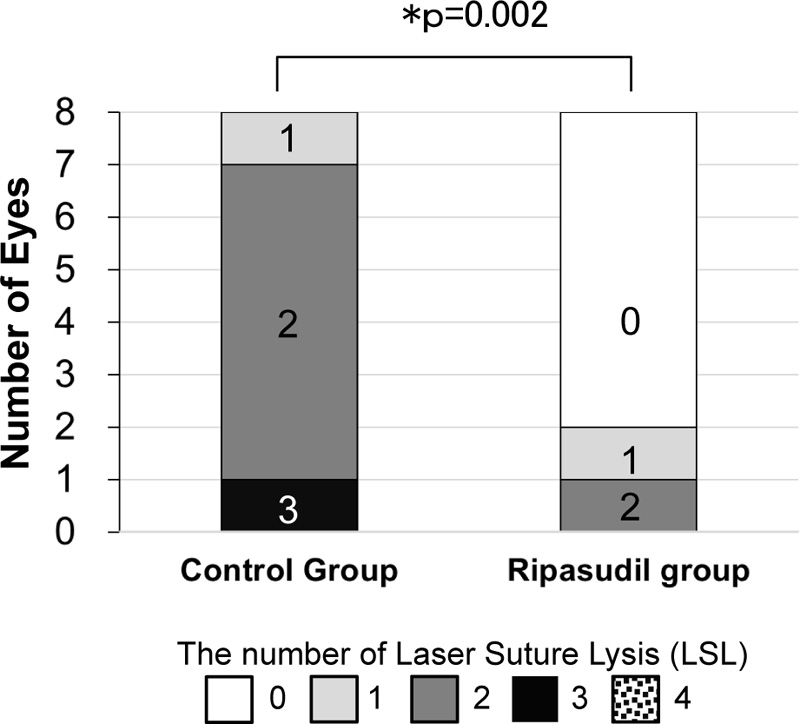
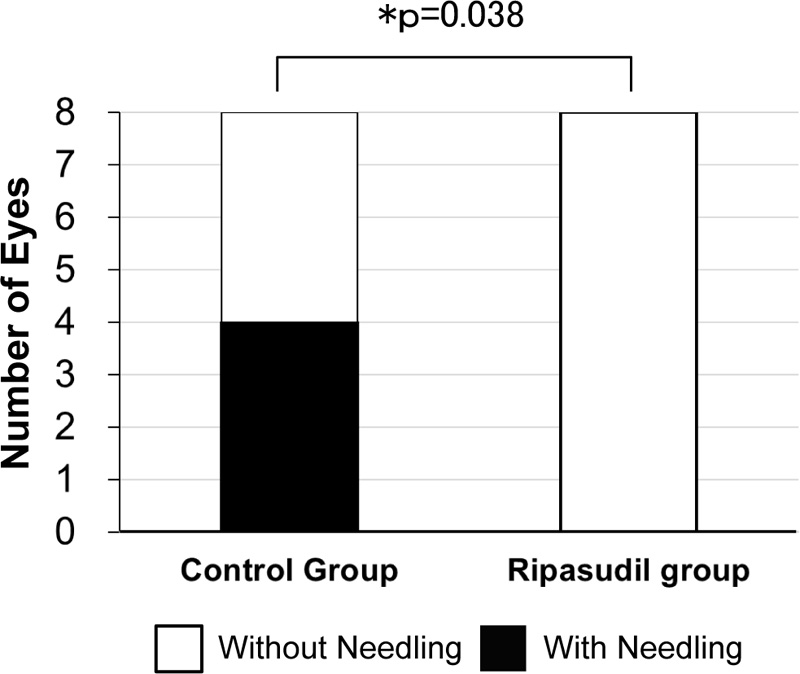
Fig. (3) shows the number of LSL procedures after trabeculectomy. The first and second LSL procedures were performed within a week in 10 of 16 eyes (62.5%), and the third was between 1 and 2 weeks in one of 16 eyes (6.3%) in both groups. Eight out of the eight eyes required LSL in the control group. In contrast, 3 out of 8 eyes required LSL in the ripasudil group. The average number of LSL procedures was higher in the control group (2.0 ± 0.5) than in the ripasudil group (0.4 ± 0.7, p = 0.002, Mann–Whitney U test) (Fig. 3).
Four of eight eyes (50.0%) required bleb revision with needling in the control group, while no eyes (0.0%) required bleb revision in the ripasudil group (p = 0.038, Fisher’s exact test) (Fig. 4).
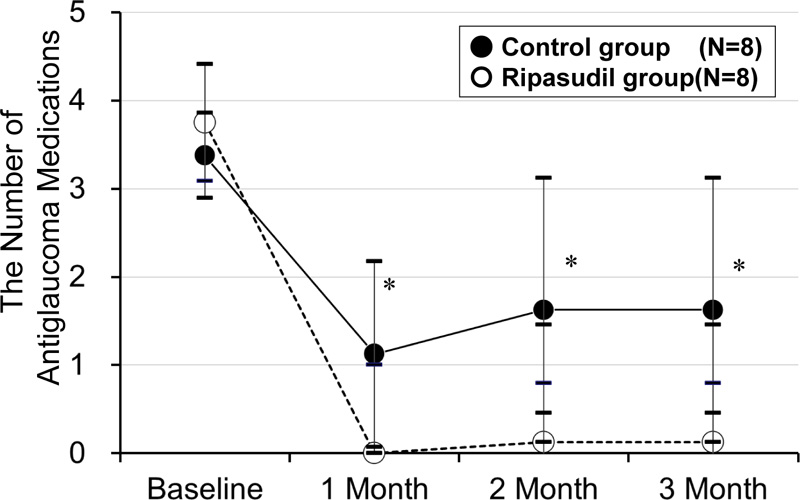
The mean number of antiglaucoma medications after trabeculectomy was higher in the control group than in the ripasudil group at 4 weeks (1.1 ± 1.1 vs. 0.0 ± 0.0, p = 0.005), at 2 months (1.6 ± 1.5 vs. 0.1 ± 0.3, p = 0.015), and at 3 months (1.6 ± 1.5 vs. 0.1 ± 0.3, p = 0.015, the Mann–Whitney U test) (Fig. 5). The multivariate analysis demonstrated that the IOP reduction rate at 3 months after surgery was associated with the use of ripasudil after the surgery (odds ratio (OR) > 100.0, p = 0.009) and baseline IOP (OR = 1.6, p = 0.006) (Table 3). The cause of uveitis did not alter the results.
| - | Multivariate analysis | ||||
| Variable | OR | (95% CI) | P value | ||
| Use of ripasudil after surgery (No=0/Yes=1) | > 100 | (17.1–> 100) | 0.009 | ||
| Duration of glaucoma (months) | 1.1 | (1.0–1.2) | 0.137 | ||
| Baseline IOP (mmHg) | 1.6 | (1.2–2.1) | 0.006 | ||
| Number of antiglaucoma eye drops before surgery | 0.1 | (0.0–51.0) | 0.408 | ||
| Lens (Phakia = 0/pseudophakia = 1) | 0.2 | (0.0–> 100) | 0.626 | ||
4. DISCUSSION
In the present study, the following main findings were obtained [1] there was a significant decrease in IOP in all patients [2], the mean percent decrease in IOP was larger in the ripasudil group than in the control group at all time visits after surgery [3], the number of LSL procedures and the rate of bleb revision by needling was higher in the control group than in the ripasudil group, and [4] multivariate analyses showed that the use of ripasudil was associated with the IOP reduction rate after the surgery. These results suggest that ripasudil effectively controlled IOP in patients with uveitic glaucoma after trabeculectomy.
We observed that IOP after trabeculectomy was lower in the ripasudil group than in the control group. This result indicates that ripasudil further reduced IOP after trabeculectomy in patients with uveitic glaucoma. A previous study demonstrated that ripasudil reduced IOP by −19.4 ± 25.1% at 1 month, −20.0 ± 27.1% at 3 months, and −23.4 ± 25.6% at 6 months from baseline patients with uveitic glaucoma [35]. This result indicates that ripasudil could further reduce IOP in patients with secondary glaucoma with high IOP, including uveitic glaucoma. Ripasudil worked well in patients with uveitic glaucoma because the baseline IOP was considerably high in eyes with uveitic glaucoma.
The number of LSL procedures was lower in the ripasudil group than in the control group. This indicated that successful filtration was achieved after incorporating ripasudil. The rate of bleb revision by needling was also lower in the ripasudil group than in the control group, suggesting that the anti-scarring activity of ripasudil might be affected by filtration surgery [29].
According to a review paper on the mechanism of ROCK inhibitors’ ability to decrease IOP by Inoue and Tanihara, ROCK inhibitors cause [1] an increase in giant vacuoles and [2] reversible changes, including decreased actin stress fibers and cell adhesion in Schlemm’s canal endothelial cells [39]. Next, the effects of ROCK inhibitors on the trabecular meshwork are [1] the relaxation of the trabecular meshwork and cytoskeleton of Schlemm’s canal cells and [2] reversal of myofibroblastic changes, reduced aqueous humor outflow resistance and increased permeability of Schlemm’s canal cells [40, 41]. Finally, ROCK inhibitors affect changes in the extracellular matrix (ECM), that is [1], the suppression of ECM production and [2] remodeling of the ECM of the trabecular meshwork and Schlemm’s canal [42-44]. These three actions of ROCK inhibitors may have reduced outflow resistance and brought our patients an IOP-lowering effect after trabeculectomy [39].
ROCK inhibitors can reduce myofibroblast differentiation and inhibit the transformation of conjunctival fibroblasts [28]. ROCK inhibitors dose-dependently inhibited human tenon fibroblast proliferation in vitro and reduced inflammation, angiogenesis, and collagen deposition in conjunctival blebs after filtration surgery in rabbits [29, 45]. The prevention of tenon adhesion to the sclera and adhesion of the scleral flap caused by tenon fibroblast proliferation leads to the maintenance of filtration blebs following glaucoma-filtering surgery. As a result, ROCK inhibitors contribute to the maintenance of blebs and might improve surgical outcomes after glaucoma filtering surgery.
Filtration activity contributing to outflow is present in only one-third of the entire trabecular meshwork in humans, as demonstrated in studies using enucleated human eyes [46, 47]. This result suggests that a very partial outflow impedes delivery to the unfiltered region of the trabecular meshwork, which may contribute most to outflow obstruction [47]. In addition, outflow obstruction in two-thirds of the entire trabecular meshwork may diminish the effectiveness of anti-glaucoma drugs [47]. Interestingly, in studies of aqueous outflow dynamics, ROCK inhibitors promoted aqueous humor outflow in the trabecular meshwork of bovine [48, 49] and monkey-excised eyes [50]. ROCK inhibitors increase outflow in the trabecular meshwork, and the increase in outflow volume is structurally correlated with the separation between the juxtacanalicular tissue and inner wall of the aqueous plexus and the size of Schlemm's canal in both bovine [48] and monkey eyes [51]. These results indicate that ROCK inhibitors increase outflow in the non-filtering portions of the trabecular meshwork, resulting in an increased outflow volume throughout Schlemm's canal. This mechanism may also be involved in Schlemm's canal after trabeculectomy. As a result, ROCK inhibitors might further promote IOP reduction after glaucoma-filtering surgery in our patients.
Furthermore, ripasudil has been reported to significantly reduce IOP and aqueous flare in inflammatory and corticosteroid-induced glaucoma associated with uveitis, suggesting that ripasudil may reduce ocular inflammation associated with uveitis.
The present study had several limitations. First, the sample size was too small due to the clinically limited number of patients undergoing surgery for secondary glaucoma available for research. The small sample size limited the statistical power of the study. Another limitation was that MMC was not used during trabeculectomy. If MMC was used in the current study, IOP control could have been good, even in the control group without ripasudil.
CONCLUSION
In conclusion, with direct and indirect effects, ripasudil may have greatly reduced IOP after glaucoma filtering surgery. The direct effect of ripasudil is to promote the outflow of aqueous humor by relaxing the trabecular meshwork and Schlemm’s canal, whereas the indirect effect is to maintain the filtering function by suppressing subconjunctival fibrosis and preventing fibrosis after trabeculectomy. Ripasudil may effectively control postoperative IOP after filtration surgery for secondary glaucoma. Further research is needed to investigate the long-term course of the IOP-lowering effects of ripasudil after glaucoma surgery.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was performed following the Declaration of Helsinki. Our Institutional Review Board approved this study (#Teirin 18-203). The present study was registered as UMIN000044345 by the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) in Japan.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans used were per the Institutional Review Board and the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Informed consent has been obtained from the participants involved.
STANDARDS OF REPORTING
Consort guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant number 20H04347) to Tatsuya Mimura, MD. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
CONFLICT OF INTEREST
Tatsuya Mimura is EIC of Open Ophthalmology Journal.
ACKNOWLEDGEMENTS
Declared none.


