All published articles of this journal are available on ScienceDirect.
Optic Disc Infiltration as a First Sign of Acute Lymphoblastic Leukemia Relapse: A Case Report
Abstract
Introduction
This case report presents a unique manifestation of relapsed B-cell Acute Lymphoblastic Leukemia (ALL) involving the ocular system. This study contributes to the scientific literature by providing documented evidence of ocular complications, specifically central retinal vein occlusion (CRVO), central retinal artery occlusion (CRAO), and optic nerve infiltration, in a patient experiencing the relapse of ALL.
Case Presentation
A 17-year-old male with a history of B-cell ALL presented with decreased vision in the right eye. Clinical examination revealed CRVO, CRAO, and optic nerve infiltration by leukemic cells. Imaging studies confirmed the presence of a retro-orbital mass and subretinal choroidal involvement. Bone marrow aspiration and biopsy confirmed relapsed of ALL. The patient was subsequently referred for further management.
Conclusion
This case highlights the importance of considering ocular manifestations, such as CRVO, CRAO, and optic nerve infiltration, in patients with relapsed ALL. Ophthalmological evaluation should be included in the follow-up and management of ALL patients to detect and manage ocular complications promptly. Early recognition and treatment of ocular manifestations can improve outcomes and quality of life for these patients.
1. INTRODUCTION
Leukemia is characterized by aberrant proliferation and differentiation of leukocytes. When this process involves the lymphoid lineage, it presents as acute lymphoblastic leukemia (ALL). The severity and timing of disease onset are determined by the proportion of leukemic blasts within the affected cell population. Specifically, a diagnosis of ALL is established when the percentage of blasts exceeds 20% in either the peripheral blood or bone marrow [1].
Therapeutic interventions for ALL have progressed considerably over time, resulting in a current cure rate of 80%. Despite the high rate of complete remission achieved with current treatment, relapse of the disease remains a significant concern, with a relapse rate of approximately 20% [2].
Relapse rates in ALL were shown to vary depending on a range of factors, including treatment modality, age at diagnosis, and other clinical features. The central nervous system (CNS), bone marrow, and testes are the most common sites of relapse in ALL, with rates of 30%, 30%, and 10%, respectively. Relapse can also occur in other extramedullary sites, such as the lymph nodes, spleen, liver, and eyes, although the incidence of relapse at these sites is typically less than 10% [3].
The patient might complain of vision loss and certain signs could be noted in the ophthalmological examination such as frosted branch angiitis, neovascular glaucoma, retinal detachment, leukemic infiltrates in the optic disc and peripapillary retina, hypopyon in the anterior segment, secondary glaucoma and corneal swelling, and heterogeneous enhancing sheet-like tissue along the retinochoroidal region associated with optic nerve thickening [4]. These ocular manifestations can arise from a range of mechanisms, including direct infiltration of leukemic cells, disruption of the blood-retina barrier, and alterations in the ocular microenvironment [4].
2. CASE PRESENTATION
A 17-year-old male patient was referred to King Saud Medical City (KSMC) in Riyadh, Saudi Arabia. He was complaining of abdominal pain, symptomatic anemia, epistaxis, and petechial skin rash. His complete blood count (CBC) suggested pancytopenia, peripheral blood smear was done and showed blast cells. Bone marrow aspiration was done in June 2019 and it showed 40% blast cells and bone marrow biopsy showed 90% cellularity and focal and interstitial infiltration of blast cells, the patient was diagnosed with B-cell acute lymphoblastic leukemia. The central nervous system was negative as the cerebrospinal fluid (CSF) analysis had no malignant cells, and BCR/ABL was negative. The patient received adolescents and Young Adults' 2015 chemotherapy protocol, induction phase, and supportive therapy. Bone marrow aspiration biopsy was done on day 14 and day 28 post-chemotherapy, where morphological remission was achieved. The patient completed the adolescents' and Young Adults' 2015 chemotherapy protocol in August 2022 and was in remission until March 2023, when he developed a decrease in vision during 1 month and he was referred to us for this new complaint. On presentation, the visual acuity of the patient was: no light perception (NLP) in the right eye, mild-to-moderate proptosis with positive relative afferent pupillary defect (RAPD), and restriction in eye movement. Fundus examination of the right eye showed a picture of central retinal vein occlusion (CRVO)and central retinal artery occlusion (CRAO) with defuse retinal hemorrhage, edema and optic disc swelling while the left eye was normal with 20/20 vision. Large prelaminar optic nerve infiltration by white leukemic cells is apparent in the fundus photo (Fig. 1). The patient was referred to hematology and for urgent magnetic resonance imaging (MRI) of the orbit with intravenous contrast.

Fundus photo shows a massive infiltration of the optic disc obscuring the optic disc margins, also multiple areas of retinal hemorrhages all over the retina in addition to small areas of retinal infiltrations.
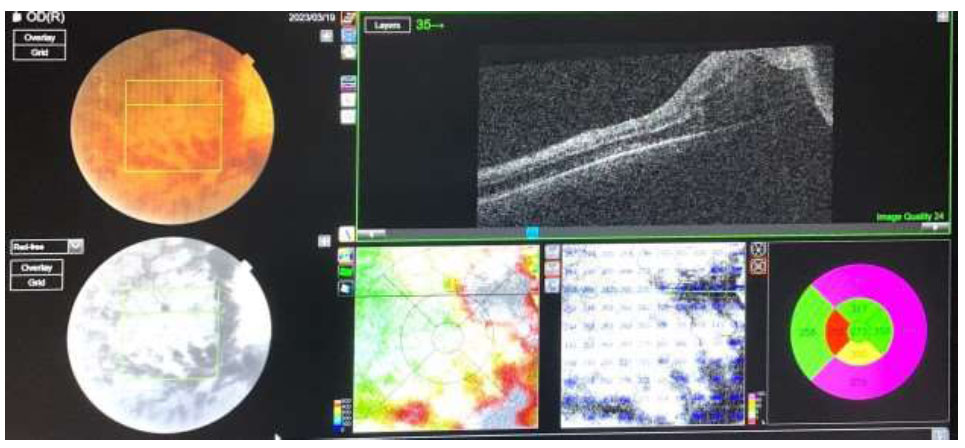
OCT scan shows CRAO and CRVO.
The Optical Coherence Tomography scan (OCT) suggested predominant features of CRVO including thickening of the macular area due to diffuse outer retinal edema with loss of foveal contour, few features of CRAO including disorganization, loss of inner retinal architecture in the foveal and parafoveal area, and denoting atrophy of inner retinal layers (Fig. 2).
In fluorescein angiography (FFA), there was a complete absence of filling of fluorescein in both arterial and venous phases suggesting CRAO (Fig. 3).
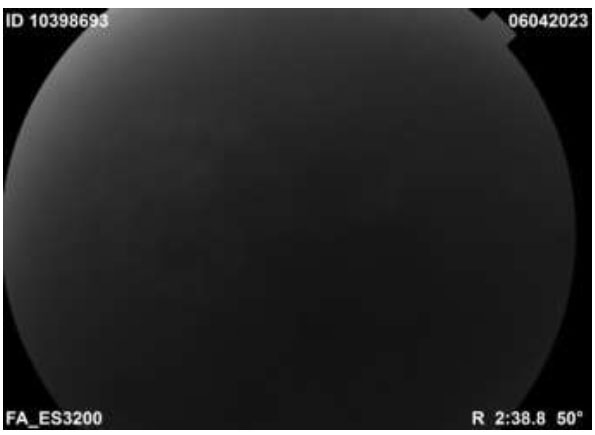
Complete absence of filling in arterial and venous phases of choroidal and retinal circulation in FFA.
MRI orbit with contrast to 23/3/2023 suggested a right infraorbital soft tissue enhancing mass surrounding the whole course of the optic nerve in the retro-orbital part, and subretinal choroidal involvement, the right superior ophthalmic vein is likely thrombosed.
The patient was admitted for evaluation, where bone marrow aspiration and biopsy were done on 29/3/2023, the sample was consistent with precursor B-cell Acute Lymphoblastic Leukemia (Relapsed). Subsequently, the patient was referred to King Fahad Medical City in Riyadh for further management.
Ethical approval has been obtained from the institutional review board at KSMC in addition to written informed consent from the patient.
3. DISCUSSION
Leukemia is a type of cancer that affects the blood and bone marrow, and it can present with a variety of symptoms and signs depending on the tissue involved [5]. The disease can spread to multiple organs and tissues, including the retina, optic nerve, choroid, lid, and intraorbital tissues, leading to a range of ocular manifestations [6]. When leukemia infiltrates the optic disc, it can be an early sign of recurrence and consequently drop in vision. The degree of vision loss can vary from mild, where the patient may not even notice it, to severe vision loss [7].
Diagnosing the recurrence of leukemia in patients can be challenging, especially in regard to identifying the cause of a decrease in vision. There are multiple factors that can contribute to vision loss in leukemia patients, and it is important to rule out any other potential causes before making a definitive diagnosis [8]. Factors such as infections and inflammations can also contribute to vision loss in leukemia. Therefore, it is crucial to determine whether the decrease in vision is due to a recurrence of leukemia or an adverse effect of the treatment [9].
Fortunately, the finding of leukemic optic nerve infiltration is quite obvious due to the large mass with clear borders accompanied by dilated, tortured veins and associated with multiple inter-retinal hemorrhages. The optic nerve is usually the place for the leukemic cells to invade due to the poor efficiency of systemic chemotherapy and intrathecal chemotherapy [10].
The most presenting signs in patients who had recurrence were headache (32.8%), followed by facial palsy (27.6%) and then vomiting (25%). However, some patients were discovered incidentally with no symptoms(13.1%). Around 6.5% of patients complain of a sudden drop in vision as the first signs of early recurrence [11]. Involvement of the optic nerve head can be prelaminar which is associated with fluffy infiltration of the optic discas observed in our case or reterolamier nerve which appears as papilledema. With such a large infiltration of the optic nerve head in our patient, we observed complete blockage of filling in choroid, retinal arteries and veins by fluorescein as a result of the direct pressure of the mass on the blood flow [12].
CONCLUSION
Optic nerve infiltration by leukemic cells can manifest as an early sign of relapse in acute lymphoblastic leukemia (ALL) patients. This case highlights the importance of considering ocular complications like central retinal vein occlusion, central retinal artery occlusion, and optic nerve infiltration during follow-up of ALL patients. Early recognition of these ocular manifestations through fundus examination and imaging is crucial for prompt management and improved patient outcomes. Timely ophthalmological evaluation should be included in the comprehensive care of ALL patients to detect vision and life-threatening complications.
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to itssubmission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ALL | = Acute Lymphoblastic Leukemia |
| CRVO | = Central Retinal Vein Occlusion |
| CRAO | = Central Retinal Artery Occlusion |
| CNS | = Central Nervous System |
| KSMC | = King Saud Medical City |
| CBC | = Complete Blood Count |
| CSF | = Cerebrospinal Fluid |
| RAPD | = Relative Afferent Pupillary Defect |
| NLP | = No Light Perception |
| MRI | = Magnetic Resonance Imaging |


