All published articles of this journal are available on ScienceDirect.
Evaluation of Retinal Nerve Fiber Layer Thickness Using Optical Coherence Tomography in the Western Region of Saudi Arabia
Abstract
Background
The Retinal Nerve Fiber Layer (RNFL) is crucial for diagnosing and monitoring optic nerve disease and physiological thinning. Optical Coherence Tomography (OCT) is an imaging technique that provides real-time scans. This procedure is used for measuring RNFL thickness and macular thickness in chorioretinal pathologies.
Objective
This study aimed to determine RNFL thickness in healthy individuals across different age groups using OCT scans.
Methods
A retrospective cross-sectional study was conducted at King Abdulaziz University Hospital in Jeddah, Saudi Arabia. The medical records of 257 patients, 105 males and 152 females from the clinic specialized in glaucoma were reviewed between October 2022 and October 2023. The analyzed variables included age, gender, and RNFL thickness.
Results
The study included participants aged 18 to 84 years, with an average age of 48.11 years. Females comprised the majority (59.1%) of the subjects compared to males (40.9%). The results indicated a significant association between age and RNFL thickness in various eye regions. Specifically, the superior, inferior, and temporal areas decreased in thickness with advancing age, particularly after 40. The most pronounced age-related changes were observed in the superior and inferior regions. Furthermore, females displayed a thicker RNFL in their right eye than males. The temporal and nasal regions showed a negative correlation with age, indicating a gradual but still noteworthy reduction in thickness in these areas.
Conclusion
This study aimed to establish a normative database contributing to the increase in quality of care in ophthalmology in Jeddah, Saudi Arabia, providing valuable guidance in diagnosing, managing, and researching glaucoma, ultimately improving patient outcomes in the region.
1. INTRODUCTION
The Retinal Nerve Fiber Layer (RNFL) is located between the inner retinal limiting membrane, which serves as the basal lamina of the Muller cells, and the retinal ganglion cell layer [1]. The evaluation of RNFL is essential because it is a crucial tool in diagnosing and monitoring the progression of optic nerve anomalies, diseases, or even normal physiological thinning [1, 2]. Various imaging devices, including wide-angle fundus photographs [3], confocal scanner, laser tomography [4], scanning laser polarimetry, and Optical Coherence Tomography (OCT) [1], can be employed to examine RNFL thickness. OCT, a non-contact medical imaging device widely used for studying chorioretinal pathologies [5-7], offers real-time, in vivo scans and measurements of ocular tissues, facilitating the assessment of RNFL and macular thickness [2, 8, 9]. The diagnostic process using OCT typically involves comparing the RNFL thickness of patients to a built-in normative RNFL thickness database [10], which is essential for different subsets of the population, given the reported ethnic variations in RNFL thickness [8, 11, 12]. A previous study conducted in India evaluated the RNFL thickness of the Indian population based on normative data, considering factors, such as age and RNFL thickness of other ethnic groups. The findings indicated that Indian patients generally showed increased RNFL thickness than Caucasians [13]. Nevertheless, a normative OCT database in Saudi Arabia remains scarce. Therefore, this study aimed to determine the RNFL thickness among healthy individuals in the Saudi population across various age groups [8, 10].
2. MATERIALS AND METHODS
This retrospective cross-sectional study was conducted at King Abdulaziz University Hospital in Jeddah, Saudi Arabia, (the medical records of 257 patients were reviewed 105 patient were males and 152 females) with the primary objective of determining the RNFL thickness among healthy individuals in the Saudi population across various age groups. The study encompassed individuals aged 18 and above, exhibiting normal ocular tension and no history of previous interventions. Ethical approval was obtained from the research ethical committee at King AbdulAziz University, Jeddah, Saudi Arabia, with reference number 146-23.
The investigation involved the review of medical records from the clinic specialized in glaucoma, covering the period from October 2022 to October 2023, with a total of 257 patients. Age and gender information was collected, and RNFL thickness was assessed using Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, California) RNFL and disc scans. Patients were present for vision screening or due to complaints of dry eye or red eye.
Exclusion criteria included all glaucoma patients, as well as individuals with diabetic retinopathy and those who had undergone laser treatment. The data have been presented as the mean ± Standard Deviation (SD) with minimum and maximum values for parametric parameters and as frequency (%) for categorized data. We used SPSS version 22 (Statistical Package for Social Sciences, IBM Corp., Armonk, New York, USA) for statistical analysis. The normality of value distribution was assessed using the Shapiro–Wilk test. One-way ANOVA was employed for data analysis, followed by Tukey's test for between-group comparisons. Pearson correlation was utilized to assess the correlation between age and RNFL, with statistical significance defined as p < 0.05.
3. RESULTS
The participants were 18–84 years old, with a mean age of 48.11 years. Most participants belonged to the age groups of 18–39 years (36.6%), followed by 40–59 years (31.9%), and ≥60 years (31.5%). Females constituted the majority of participants at 59.1%, compared to males at 40.9% (Table 1).
| Characteristics | Value |
|---|---|
| Age (Years) | 48.11 ± 16.58 (18.00–84.00) |
| 18–39 years | 94 (36.6%) |
| 40–59 years | 82 (31.9%) |
| ≥60 years | 81 (31.5%) |
| Gender | - |
| Male | 105 (40.9%) |
| Female | 152 (59.1%) |
The superior RNFL thickness for the right and left eyes ranged from 69.00 to 226.00 and 65.00 to 180.00, with mean values of 119.28 and 124.24, respectively. Notably, there were significant variations in superior RNFL thickness between different age groups (F = 8.422, p < 0.0001, and F = 12.223, p < 0.0001). Specifically, the superior RNFL thickness of both eyes was significantly higher in the 18–39 age group compared to the 40–59 years and ≥60 years groups (p = 0.030, p < 0.0001, and p = 0.005, p < 0.0001). The inferior RNFL thickness for the right and left eyes ranged from 76.00 to 237.00 and 66.00 to 200.00, with mean values of 130.30 and 130.08, respectively.
Differences in inferior RNFL thickness were observed between different age groups (F = 12.805, p < 0.0001, and F = 11.307, p < 0.0001). Similar to the superior RNFL, the inferior RNFL thickness of both eyes was significantly higher in the 18–39 age group compared to the 40–59 years and ≥60 years groups (p = 0.010, p < 0.0001, and p = 0.005, p < 0.0001). The temporal RNFL thickness for the right and left eyes ranged from 27.00 to 109.00 and 29.00 to 110.00, with mean values of 70.78 and 68.63, respectively. Significant changes in temporal RNFL thickness were observed between different age groups (F = 5.265, p = 0.006, and F = 8.665, p < 0.0001). The temporal RNFL thickness of both eyes was significantly higher in the 18–39 age group compared to the 40–59 years and ≥60 years groups (p = 0.007, p = 0.046, and p < 0.0001, p = 0.009). The nasal RNFL thickness for the right and left eyes ranged from 41.00 to 115.00 and from 16.00 to 153.00, with mean values of 75.48 and 74.90, respectively. There were insignificant changes in nasal RNFL thickness in the right eye between different age groups (F = 2.797, p = 0.063). However, significant changes were noted for the left eye (F = 4.409, p = 0.013), with the nasal RNFL thickness being significantly higher in the 18–39 age group compared to the ≥60 years group (p = 0.010) (Table 2).
The superior RNFL thickness of the right eye was significantly higher in females than in males (F = 4.061, p = 0.045). Meanwhile, there were insignificant changes between males and females regarding left eye superior (F = 0.287, p = 0.592), right eye inferior (F = 1.969, p = 0.162), left eye inferior (F = 0.561, p = 0.455), right eye temporal (F = 1.298, p = 0.256), left eye temporal (F = 1.177, p = 0.279), right eye nasal (F = 0.069, p = 0.794), and left eye nasal (F = 0.166, p = 0.684) (Table 3).
| RNFL | All (n = 257) |
Aged 18–39 (n = 94) |
Aged 40–59 (n = 82) |
Aged ≥60 (n = 814) |
F | Significance |
|---|---|---|---|---|---|---|
| - | Right eye | - | - | |||
| Superior | 119.28 ± 18.95 | 125.14 ± 19.34 | 118.00 ± 18.03 | 113.85 ± 17.71 | 8.422 | 0.0001 |
| - | 69.00–226.00 | 70.00–226.00 | 77.00–164.00 | 69.00–150.00 | - | - |
| Significance | - | - | 1p = 0.030 | 1p < 0.0001,2p = 0.323 | - | - |
| - | Left eye | - | - | |||
| Superior | 124.24 ± 17.50 | 130.64 ± 16.59 | 122.67 ± 16.03 | 118.25 ± 17.73 | 12.223 | 0.0001 |
| - | 65.00–180.00 | 88.00–180.00 | 91.00–165.00 | 65.00–157.00 | - | - |
| Significance | - | - | 1p = 0.005 | 1p < 0.0001,2p = 0.219 | - | - |
| - | Right eye | - | - | |||
| Inferior | 130.30 ± 20.70 | 137.89 ± 22.30 | 229.10 ± 18.26 | 122.79 ± 18.20 | 12.805 | 0.0001 |
| - | 76.00–237.00 | 76.00–237.00 | 87.00–171.00 | 86.00–161.00 | - | - |
| Significance | - | - | 1p = 0.010 | 1p < 0.0001,2p = 0.107 | - | - |
| - | Left eye | - | - | |||
| Inferior | 130.08 ± 20.25 | 137.31 ± 20.54 | 128.07 ± 18.53 | 123.56 ± 19.22 | 11.307 | 0.0001 |
| - | 66.00–200.00 | 90.00–200.00 | 66.00–174.00 | 76.00–167.00 | - | - |
| Significance | - | - | 1p = 0.005 | 1p < 0.0001,2p = 0.308 | - | - |
| - | Right eye | - | - | |||
| Temporal | 70.78 ± 12.28 | 73.96 ± 11.07 | 68.37 ± 11.78 | 69.57 ± 13.41 | 5.265 | 0.006 |
| - | 27.00–109.00 | 49.00–109.00 | 46.00–96.00 | 27.00–105.00 | - | - |
| Significance | - | - | 1p = 0.007 | 1p = 0.046,2p = 0.801 | - | - |
| - | Left eye | - | - | |||
| Temporal | 68.63 ± 11.42 | 72.32 ± 10.13 | 65.68 ± 11.69 | 67.30 ± 11.53 | 8.665 | 0.0001 |
| - | 29.00–110.00 | 49.00–110.00 | 29.00–91.00 | 36.00–98.00 | - | - |
| Significance | - | - | 1p = 0.0001 | 1p = 0.009,2p = 0.624 | - | - |
| - | Right eye | - | - | |||
| Nasal | 75.48 ± 12.30 | 77.15 ± 13.24 | 76.14 ± 12.84 | 72.90 ± 10.17 | 2.797 | 0.063 |
| - | 41.00–115.00 | 41.00–115.00 | 46.00–109.00 | 52.00–106.00 | - | - |
| Significance | - | - | 1p = 0.850 | 1p = 0.059, 2p = 0.209 | - | - |
| - | Left eye | - | - | |||
| Nasal | 74.90 ± 14.27 | 77.55 ± 16.51 | 75.43 ± 11.69 | 71.23 ± 13.19 | 4.409 | 0.013 |
| - | 16.00–153.00 | 49.00–153.00 | 51.00–112.00 | 16.00–113.00 | - | - |
| Significance | - | - | 1p = 0.578 | 1p = 0.010,2p = 0.143 | - | - |
| RNFL | Male (n = 104) |
Female (n = 152) |
F | Significance |
|---|---|---|---|---|
| Right eye superior | 116.41 ± 18.82 | 121.24 ± 18.84 | 4.061 | 0.045 |
| - | 69.00–166.00 | 70.00–226.00 | - | - |
| Left eye superior | 123.52 ± 17.97 | 124.72 ± 17.23 | 0.287 | 0.592 |
| - | 65.00–167.00 | 66.00–180.00 | - | - |
| Right eye inferior | 128.11 ± 20.05 | 131.80 ± 21.07 | 1.969 | 0.162 |
| - | 76.00–185.00 | 89.00–237.00 | - | - |
| Left eye inferior | 128.92 ± 19.94 | 130.86 ± 20.54 | 0.561 | 0.455 |
| - | 79.00–181.00 | 66.00–200.00 | - | - |
| Right eye temporal | 69.72 ± 12.23 | 71.50 ± 12.30 | 1.298 | 0.256 |
| - | 27.00–105.00 | 32.00–109.00 | - | - |
| Left eye temporal | 67.69 ± 10.62 | 69.27 ± 11.92 | 1.177 | 0.279 |
| - | 36.00–91.00 | 29.00–110.00 | - | - |
| Right eye nasal | 75.24 ± 11.94 | 75.65 ± 12.58 | 0.069 | 0.794 |
| - | 41.00–100.00 | 43.00–115.00 | - | - |
| Left eye nasal | 74.47 ± 13.82 | 75.21 ± 14.61 | 0.166 | 0.684 |
| - | 30.00–153.00 | 16.00–136.00 | - | - |
| Parameters | r (significance) |
|---|---|
| Right eye superior RNFL thickness | −0.228 (p < 0.0001) |
| Left eye superior RNFL thickness | −0.282 (p < 0.0001) |
| Right eye inferior RNFL thickness | −0.288 (p < 0.0001) |
| Left eye inferior RNFL thickness | −0.271 (p < 0.0001) |
| Right eye temporal RNFL thickness | −0.175 (p = 0.005) |
| Left eye temporal RNFL thickness | −0.183 (p = 0.003) |
| Right eye nasal RNFL thickness | −0.125 (p = 0.046) |
| Left eye nasal RNFL thickness | −0.183 (p = 0.003) |
Correlations between age and RNFL are shown in Table 4 and Figs. (1-4). There were significant negative correlations between age and right eye superior (r = −0.228, p < 0.0001) and left eye superior (r = −0.282, p < 0.0001), right eye inferior (r = −0.288, p < 0.0001) and left eye inferior (r = −0.271, p < 0.0001), right eye temporal (r = −0.175, p = 0.005) and left eye temporal (r = −0.183, p = 0.003), right eye nasal (r = −0.125, p = 0.046) and left eye superior (r = −0.183, p = 0.003).
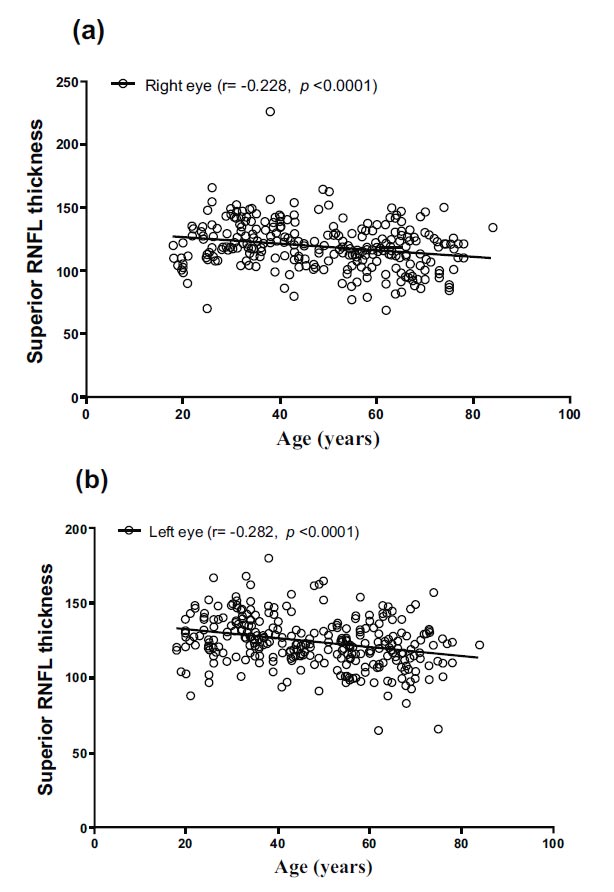
Correlation between age and superior RNFL thickness in (a) right and (b) left eyes.
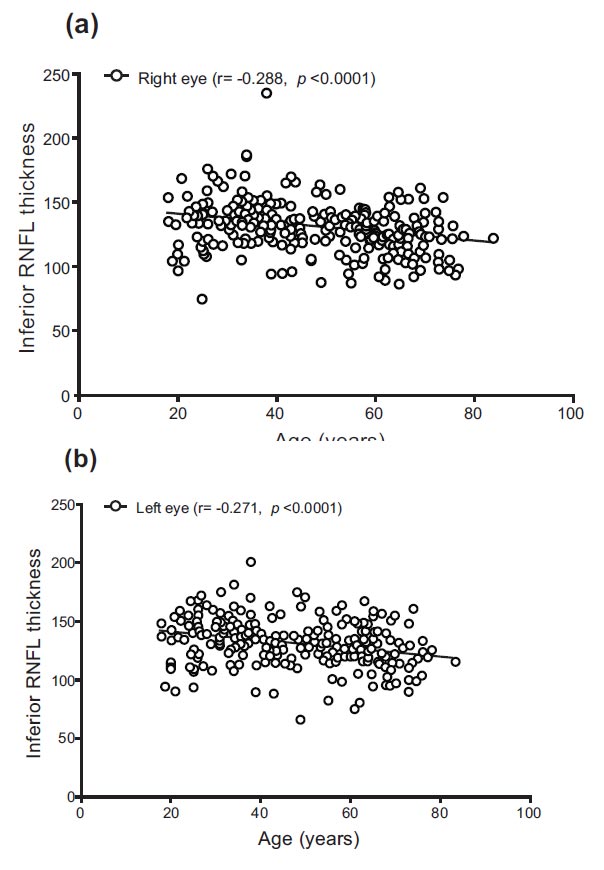
Correlation between age and inferior RNFL thickness in (a) right and (b) left eyes.
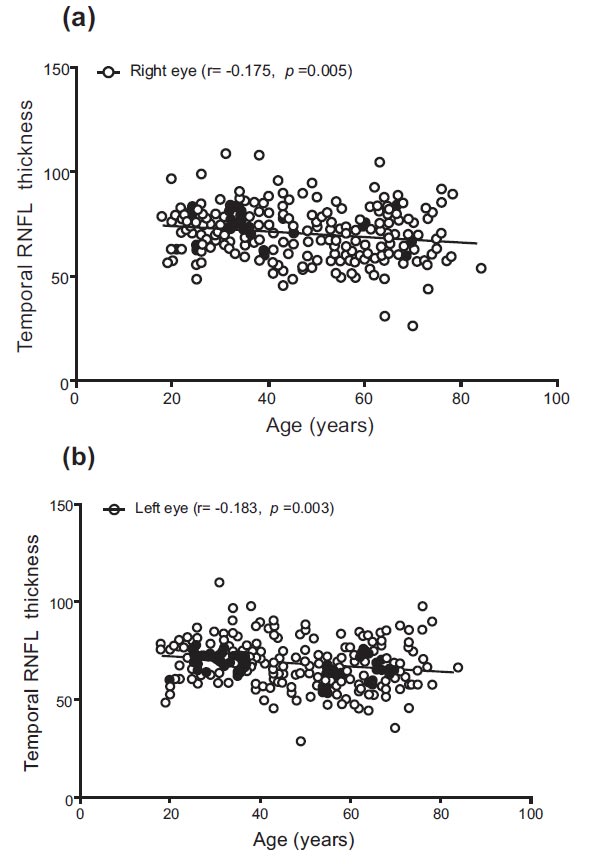
Correlation between age and temporal RNFL thickness in (a) right and (b) left eyes.
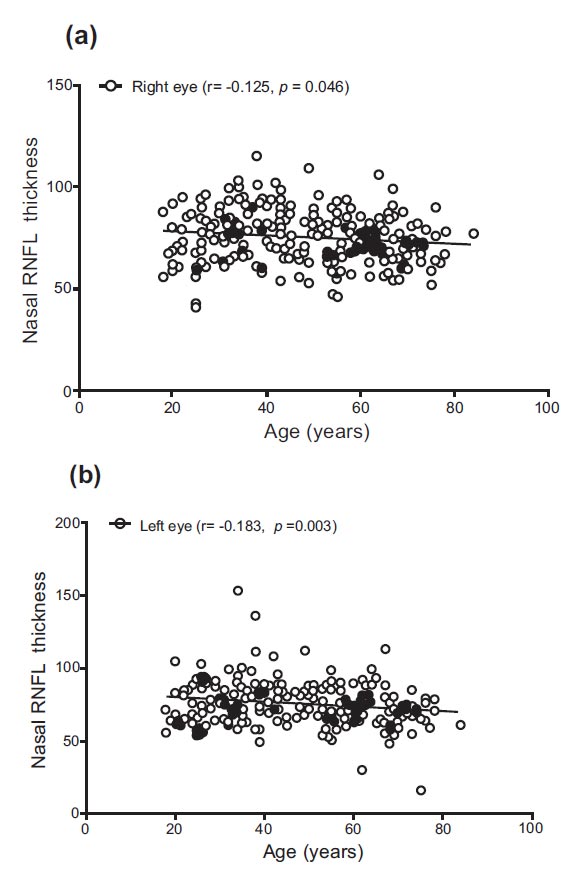
Correlation between age and nasal RNFL thickness in (a) right and (b) left eyes.
4. DISCUSSION
The study's demographic distribution encompassed a broad spectrum of the adult population, with participants aged 18 to 84, accounting for a mean age of 48.11 years. The prevalence of younger participants, particularly in the 18–39 age range, coupled with a slightly higher proportion of females (59.1%), has provided a diverse sample for exploring ocular health across various age groups and genders.
Aging significantly impacts RNFL thickness. Results have revealed a consistent decline in RNFL thickness in the superior, inferior, and temporal areas with increasing age, especially beyond 40 years of age. This pattern aligns with research on Age-related Macular Degeneration (AMD) conducted by Trinh et al. [14], emphasizing age-related variations in retinal layer thickness. Their study underscored the efficacy of OCT in detecting early aging-related changes in the retina, a point emphasized by Shin et al. [15] in their study on OCT measures in AMD.
Gender's impact on RNFL thickness, assessed by OCT, provides valuable insights into ocular health. In this study, females exhibited significantly thicker RNFL in their right eyes than males, providing a novel perspective on gender-specific ocular traits [16]. This finding diverges from Najeeb et al.'s study [17], where females tended to have thinner inner and outer retinal layers. This variation may be attributed to ethnic or demographic differences, emphasizing the collective influence of genetic, environmental, and physiological factors on RNFL thickness. Gender differences in RNFL thickness were not significant in other aspects, aligning with Cubuk, Sahinoglu, and Keskek's [8] study on a Turkish population, indicating that gender-specific variations in the RNFL thickness may only exist in some areas of the retina. The study's results may contribute to the growing body of research on gender differences in ocular characteristics, underscoring the importance of considering gender in evaluating ocular health. These findings are crucial for advancing personalized approaches to diagnosing and treating eye conditions, especially those exhibiting gender-specific patterns.
There has been a significant relationship noted between age and RNFL thickness in different parts of the eye. The superior and inferior parts manifested the most noticeable signs of aging, emphasizing the importance of vigilant ocular health monitoring, especially in older individuals. Although less pronounced, the temporal and nasal parts also negatively correlated with age, indicating a less severe yet significant decrease in RNFL thickness in these regions.
Clinical evaluation must account for these retina region-specific differences in RNFL physiological thinning associated with aging. The findings of this study underscore the need for age-appropriate standards in diagnosing and treating ocular diseases, particularly glaucoma, where RNFL thickness is an important indicator. Ocular health evaluation and treatment strategies for the elderly can be significantly improved using this detailed knowledge of how aging affects certain parts of the RNFL. The identified pattern of RNFL physiological thinning aligns with the observations reported by Trinh et al. [14]. Their study revealed significant physiological thinning in the RNFL of eyes affected by AMD. Additionally, the study by Feuer et al. [18] on age-related variations in RNFL thickness reinforced these findings, highlighting the reduction to not be uniform across the optic disc, with more pronounced changes detected in the superior region. These results underscore the progressive decline in RNFL thickness with age, emphasizing the importance of considering these changes in clinical assessment of ocular health. Furthermore, the study has emphasized the necessity of employing age-adjusted criteria in ocular evaluation, particularly in identifying and tracking conditions, such as glaucoma, where RNFL thickness is a critical metric. Choi and Seol's [15] research on the impact of cataracts on RNFL thickness accentuated the potential influence of other ocular conditions on these measurements. Therefore, a comprehensive eye examination should account for age-related changes and conditions impacting RNFL thickness. The study by Shin et al. [16] verified the reliability and accuracy of OCT technology, confirming its validity in identifying and monitoring changes over time.
CONCLUSION
The assessment of RNFL thickness is essential in ophthalmic examinations, especially in diagnosing glaucoma. Consequently, exploring diverse variables and establishing their correlation with RNFL thickness can enhance patient care in ophthalmology practice. This can be achieved by creating a normative database utilizing Cirrus HD-OCT for patients in Jeddah, Saudi Arabia. Such a database can serve as a valuable guideline in diagnosing, managing, and further researching glaucoma.
AUTHORS' CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to itssubmission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ANOVA | = Analysis of variance |
| OCT | = Optical coherence tomography |
| RNFL | = Retinal nerve fiber layer |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the research ethics committee of King Abdulaziz University Hospital, Saudi Arabia, with approval number 146-23.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of1975, as revised in 2013


