All published articles of this journal are available on ScienceDirect.
Associations between Retinal and Choroidal Vascularization Parameters and Brachial Artery Flow-mediated Dilation in Type 2 Diabetes and Healthy Controls
Abstract
Introduction
Patients with type 2 Diabetes Mellitus (DM) are a group at an increased cardiovascular risk, which is associated with impaired vascular endothelial function. The aim of our study was to determine whether retinal and choroidal vascularization parameters are related to vascular endothelial function as expressed by flow-mediated vasodilatation (FMD).
Methods
Thirty-two eyes of 32 patients were included in this observational study; 15 eyes were categorized into the study group, defined as type 2 diabetic patients without diabetic retinopathy and other diabetic complications, and 17 in the healthy control group. RTVue XR Avanti optical coherent tomography angiography (angio-OCT) was used to perform OCT scans and visualize the superficial and deep retinal plexus (SCP and DCP, respectively). Using OCT image binarization, the choroidal vascularity index (CVI) was calculated. Brachial FMD was measured for each participant.
Results
There was no difference in FMD between the DM group and healthy controls (6.64 vs 5.67, p= 0.47, respectively). A positive correlation of FMD was found with the perifoveal SCP and CVI (r=0.57 and r=0.58, respectively) in the control group and with perifoveal DCP in the study group, control group, and the whole studied population (r=0.58, r=0.89, and r=0.68, respectively). In multivariate linear regression, after adjusting for age and sex, FMD was associated with the presence of hypertension (b=-0.4) and perifoveal DCP (b=0.47).
Conclusion
Retinal capillary plexus density parameters are positively associated with peripheral vascular endothelial function expressed by FMD in type 2 diabetes and healthy populations.
1. INTRODUCTION
Type 2 Diabetes Mellitus (DM) has a significant impact on the eye, as diabetic retinopathy is one of the leading causes of preventable vision loss [1]. One of the primary tissues affected by diabetes is the vascular endothelium, a layer of specialized cells lining the interior surface of blood vessels. This tissue plays a crucial role in various functions, including hemostasis, inflammation modulation, vaso- constriction, and vasodilation [2]. It is suggested that damage to the vascular endothelium is critical in the development of diabetic retinopathy and that such damage also affects the choroid [3, 4].
Optical Coherence Tomography Angiography (OCTA) is a non-invasive examination that enables the visualization of the superficial and deep retinal capillary plexus vascular densities (SCP and DCP, respectively). These vessels consist of a single layer of endothelial cells, along with pericytes and a basement membrane [5]. Visualizing the choroidal vasculature is more challenging, although it may be assessed using the Choroidal Vascularity Index (CVI), as proposed by Agrawal et al. [6].
Vascular endothelial function is also impaired in cardiovascular disease. Flow-mediated vasodilation (FMD) is widely used to assess endothelial function in peripheral arteries [7]. This phenomenon describes the vasodilation of an artery in response to increased blood flow, such as after a period of ischemia. Studies have reported that FMD of the brachial artery is an indicator of cardio- vascular risk [8].
One of the main factors contributing to FMD is the release of endothelium-derived nitric oxide (NO). It has been shown that NO modulates basal flow through the retinal and choroidal vasculature and influences the response of other vasodilatory factors, such as bradykinin, acetylcholine, and histamine [9]. Ocular hemodynamics is impaired by endothelial dysfunction because it increases the synthesis of reactive oxygen species and decreases the absorption of NO [10]. FMD has previously been studied in regard to the retinal and choroidal diseases. It has been found to be decreased in advanced diabetic retinopathy and wet age-related macular degeneration [11, 12]. These findings suggest that peripheral endothelial function measured by FMD may influence the ocular vascular system.
Identifying ophthalmic equivalents of FMD could facilitate the detection of patients with potentially increased cardiovascular risk and enhance our under- standing of the pathophysiology of diabetic eye disease. This is particularly important because the diabetic population is already at an elevated risk for cardiovascular disease, which is further exacerbated by the presence of diabetic retinopathy [13]. To the best of our knowledge, it has not yet been determined whether vascular endothelial function, as expressed by FMD, translates into retinal and choroidal vascular status in patients with type 2 diabetes mellitus.
The aim of our study is to identify which ocular vascularisation parameters (SCP, DCP, or CVI) may be related to vascular endothelial function in the peripheral circulation, expressed by FMD, in type 2 diabetic patients and healthy population.
2. METHODS
This observational study included patients with type 2 diabetes without diabetic retinopathy and healthy controls. Exclusion criteria included type 1 diabetes, age under 18 years, intraocular pressure over 21 mmHg, previous ocular treatments, retinal diseases, ocular diseases that may occlude the visual axis (corneal opacities, cataract), systemic diseases other than diabetes and hypertension (HT). Data on HbA1C levels and hypertension history were collected.
Comprehensive ophthalmological examinations were conducted for each patient, which involved slit-lamp examination of the anterior segment and fundus, best-corrected visual acuity assessment using a Snellen chart (maximum limit of 1.0), axial length measurement (ARGOS biometer, Alcon, Switzerland), and air-puff intraocular pressure measurement (TRK-2p, Topcon, Japan). Spectral-domain optical coherence tomography (SD-OCT) and angio-OCT scans of the macula (3 × 3 mm) were taken using the RTVue XR Avanti (Optovue Inc., Fremont, CA, USA). SCP and DCP were calculated using built-in software. Only the eye with the better image quality in the angio-OCT scans was selected for analysis in each participant. All measurements were performed under consistent room lighting conditions in the evening.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Wroclaw Medical University, Poland (KB-510/2022). Informed consent was obtained from all subjects involved in the study.
2.1. Flow-mediated Vasodilatation Measurement
Subjects were asked not to eat and avoid exercise, caffeine, and alcohol for 6 hours before the measurement. The test was performed in a quiet, darkened room at the same time of the day for each patient in the evening. FMD was measured in the supine position. The upper limb was rested on a gutter foam, supported on a table to be at the height of the patient's heart. First, systolic and diastolic pressures were measured, and after 10 minutes, a 5-second longitudinal recording of the brachial artery was made. The measurement was performed using a linear B-mode 4-13 MhZ linear probe ultrasound machine Aloka prosound a6 (Aloka Co. Ltd., Tokyo, Japan). The correct location of the artery was confirmed using Doppler pulse wave analysis. The cuff placed on the forearm was then inflated to a value at least 50 mmHg greater than the previously measured systolic pressure. After 5 minutes, the cuff was released, and the brachial artery was re-measured 2 minutes after cuff deflation. All measurements were taken by the same investigator.
Each measurement image quality was assessed, and a peak diameter frame with clear vessel wall boundaries was included in the analysis. The arterial diameter was measured using ImageJ (1.54G, Wayne Rasband, NIH, USA, Available at http://imagej.nih.gov/ij/docs/index.html) and the publicly available macro InteredgeDistance. Image analysis was performed by the same blinded investigator. FMD was calculated using the formula: FMD = [(maximum diameter - baseline diameter)/ baseline diameter] × 100 (%).
The accuracy and reproducibility of FMD measure- ments in this study were ensured by adhering to a standardized protocol described above. All FMD assess- ments were performed by a single trained operator, minimizing inter-operator variability. All patients were measured under consistent conditions to control for environmental and physiological factors that might affect FMD outcomes.
2.2. Choroidal Parameters Calculation
Using MATLAB (R2018a, MathWorks, Inc., Natick, MA, USA), we analyzed raw OCT files, focusing on the 3 × 3 mm area centered on the fovea. Each scan was used to create a 3D model of the choroidal space centered on the macula, with the ETDRS chart overlaid on the choroid. Choroidal thickness (CT), measured from Bruch’s membrane to the choroidoscleral junction (CSJ), was determined using an automated method that evaluates the CSJ type and visibility of ciliary vessels. Artificial intelligence/ machine learning libraries containing characteristics of CSJ types, visibility, and ciliary vessel locations ensured the reproducibility of the CSJ line. The OCT scans were converted from grayscale to binary images to determine the luminal area (LA), defined as the area of dark pixels, and the total choroidal area (TCA). The choroidal vascularity index (CVI) was then calculated using the formula: CVI = LA/TCA.
2.3. Statistical Analysis
The normality of the data distribution was evaluated using the Lilliefors test. For intergroup comparisons, the Mann–Whitney U test was used for continuous variables without a normal distribution, while the Student's t-test was applied for variables with a normal distribution and equal variances tested by Levene’s test. Spearman correlation was used to analyze how clinical and vascularization parameters affect FMD in the DM group, control group, and total population. Univariate linear regression was performed with FMD as the dependent variable and clinical and vascularization parameters as independent variables. Following this, multivariate linear regression was conducted, including all parameters that were statistically significant (p < 0.1) in the univariate regression, as well as age and sex, regardless of their significance in the univariate analysis. Categorical variables were assigned binary values and underwent sigma-restricted parameterisation. Statistical analysis was conducted using Statistica Software version 13.3 (TIBCO Statistica 1984-2017, TIBCO Software Inc.). Data in tables and figures are presented as mean. In the cases of missing data, the participant was omitted from the analysis.
The central region (-C) was defined as a 1.0 mm diameter circle around the fovea, while the parafoveal (-P) area was described by extending 2.0 mm around the central region. The values for SCP, DCP, CT, and CVI within the parafoveal quadrants were averaged, and only the eye with the better image quality was selected for analysis in each participant.
3. RESULTS
Thirty-two patients participated in the study; 15 were in the study group, and 17 were in the control group. The groups did not differ significantly in terms of sex and age (p=0.49 and p= 0.52, respectively) or other clinical characteristics (Table 1). In the diabetes group, the mean diabetes duration was 6.63 years, and the mean HbA1C level was 7.6%. There was no statistical difference in FMD between the DM and control group, despite the DM group having higher mean FMD (6.64 vs 5.67, p= 0.47, respectively). This lack of significance may be attributed to the higher standard deviation in the DM group (3.69 vs 2.67, respectively). Of the retinal and choroidal vascu- larization parameters, CVI-C and perifoveal CVI-P were significantly reduced in the study group compared to the control group (32.56 vs. 47.17 and 53.08 vs. 59.34, respectively) (Table 2 and Fig. 1).
FMD correlated with clinical characteristics, showing a negative correlation with age in the general population and the control group (r=-0.43, p= 0.01; r=-0.51, p=0.04, respectively). In the diabetic group, FMD was also negatively correlated to HT (r=-0.57, p=0.03). When examining the correlation between FMD and vascu- larisation parameters, a positive correlation was found with the SCP-P and CVI-P in the control group (r=0.57, p=0.02; r=0.58, p=0.01, respectively). Additionally, a positive correlation between FMD and DCP-P was observed in the study group, the control group, and the general population (r=0.58, p=0.04; r=0.86, p<0.001; r=0.68, p<0.001, respectively) (Table 3).
| Parameter | DM | SD | Control | SD | p | Total | SD |
|---|---|---|---|---|---|---|---|
| Age (years) | 58.20 | 11.97 | 55.06 | 15.07 | 0.52 | 56.53 | 13.58 |
| Female sex (%) | 7(46.67) | - | 10(58.82) | - | 0.49 | 17 (53.13) | - |
| SQ | 8.27 | 1.16 | 8.18 | 0.88 | 0.51 | 8.22 | 1.01 |
| BCVA | 0.94 | 0.12 | 0.97 | 0.10 | 0.30 | 0.95 | 0.11 |
| Intraocular pressure (mmHg) | 16.33 | 2.23 | 16.24 | 2.93 | 0.92 | 16.28 | 2.58 |
| Axial length (mm) | 23.57 | 0.84 | 23.39 | 0.92 | 0.60 | 23.48 | 0.87 |
| Systolic BP (mmHg) | 137.60 | 23.42 | 138.94 | 19.25 | 0.86 | 138.31 | 20.96 |
| Diastolic BP (mmHg) | 83.87 | 12.36 | 88.63 | 11.93 | 0.36 | 86.32 | 12.17 |
| Hypertension (%) | 9(60) | - | 12(70.59) | - | 0.53 | - | - |
| Diabetes duration (years) | 6.63 | 6.72 | - | - | - | - | - |
| HbA1C (%) | 7.60 | 1.52 | - | - | - | - | - |
| Parameter | DM | SD | Control | SD | p | Total | SD |
|---|---|---|---|---|---|---|---|
| FMD (%) | 6.64 | 3.69 | 5.67 | 2.67 | 0.47 | 6.13 | 3.17 |
| SCP-C | 20.73 | 5.39 | 19.12 | 5.60 | 0.41 | 19.88 | 5.48 |
| SCP-P | 48.42 | 2.95 | 49.19 | 2.78 | 0.51 | 48.83 | 2.84 |
| DCP-C | 34.17 | 6.58 | 31.73 | 9.77 | 0.52 | 33.00 | 8.16 |
| DCP-P | 52.81 | 3.81 | 52.36 | 3.69 | 1.00 | 52.60 | 3.68 |
| CT-C (μm) | 274.90 | 17.69 | 267.07 | 23.31 | 0.31 | 270.61 | 21.00 |
| CT-P (μm) | 312.06 | 12.15 | 312.00 | 12.32 | 0.95 | 312.03 | 12.04 |
| CVI-C | 32.56 | 8.89 | 47.17 | 8.66 | 0.00 | 40.32 | 11.37 |
| CVI-P | 53.08 | 4.34 | 59.34 | 3.12 | 0.00 | 56.41 | 4.85 |
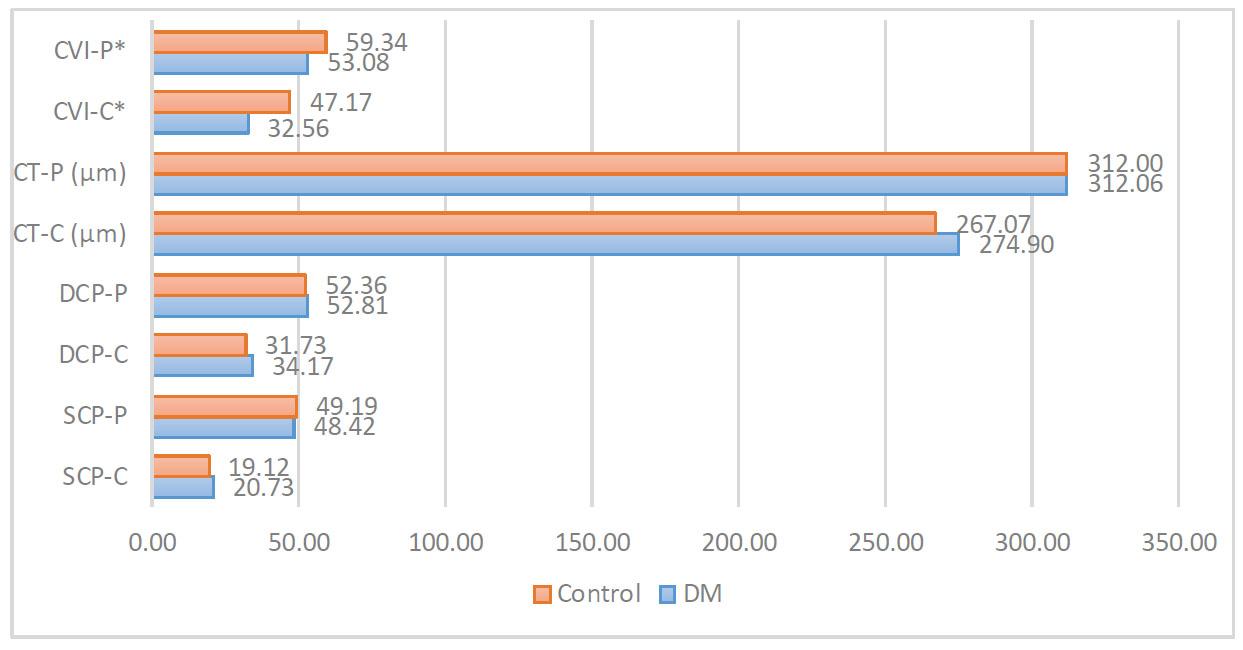
Comparison of vascularization parameters.
*, p<0.05; DM, diabetes mellitus type 2; SCP, superficial capillary plexus vascular density; DCP, deep capillary plexus vascular density; CT, choroidal thickness; CVI, choroidal vascularity index; -C, central; -P, perifoveal.
| Parameter | DM | Control | Total |
|---|---|---|---|
| Age | -0.43 | -0.51* | -0.43* |
| Male sex | 0.25 | -0.15 | 0.08 |
| Intraocular pressure | 0.32 | 0.16 | 0.23 |
| Axial length | 0.43 | 0.06 | 0.23 |
| Systolic BP | -0.21 | 0.42 | 0.11 |
| Diastolic BP | 0.04 | 0.19 | 0.09 |
| Hypertension | -0.57* | 0.05 | -0.26 |
| SCP-C | -0.11 | -0.37 | -0.21 |
| SCP-P | 0.14 | 0.57* | 0.32 |
| DCP-C | -0.16 | -0.30 | -0.23 |
| DCP-P | 0.58* | 0.86* | 0.68* |
| CT-C | 0.00 | -0.38 | -0.20 |
| CT-P | -0.33 | -0.13 | -0.19 |
| CVI-C | 0.06 | 0.00 | -0.04 |
| CVI-P | 0.23 | 0.58* | 0.21 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameter | Beta | p | CI 95% | Beta | p | CI 95% |
| Age | -0.38 | 0.03 | (-0.73, -0.04) | -0.18 | 0.38 | (-0.59, 0.24) |
| Male sex | -0.04 | 0.81 | (-0.42, 0.33) | 0.04 | 0.81 | (-0.32, 0.40) |
| Group (1-DM) | 0.16 | 0.40 | (-0.21, 0.52) | - | - | - |
| Intraocular pressure | 0.19 | 0.29 | (-0.17, 0.56) | - | - | - |
| Axial length | 0.20 | 0.31 | (-0.19, 0.58) | - | - | - |
| Systolic BP | -0.03 | 0.87 | (-0.40, 0.34) | - | - | - |
| Diastolic BP | -0.06 | 0.73 | (-0.44, 0.31) | - | - | - |
| Hypertension | -0.33 | 0.06 | (-0.68, 0.02) | -0.40 | 0.03 | (-0.75, -0.05) |
| Diabetes duration | 0.14 | 0.64 | (-0.51, 0.80) | - | - | - |
| HbA1C | -0.08 | 0.74 | (-0.54, 0.39) | - | - | - |
| SCP-C | -0.21 | 0.24 | (-0.58, 0.15) | - | - | - |
| SCP-P* | 0.36 | 0.04 | (0.01, 0.71) | - | - | - |
| DCP-C | -0.14 | 0.51 | (-0.59, 0.30) | - | - | - |
| DCP-P | 0.57 | 0.00 | (0.19, 0.94) | 0.47 | 0.03 | (0.06, 0.88) |
| CT-C | -0.24 | 0.20 | (-0.61, 0.13) | - | - | - |
| CT-P | -0.22 | 0.25 | (-0.59, 0.16) | - | - | - |
| CVI-C | -0.18 | 0.33 | (-0.54, 0.19) | - | - | - |
| CVI-P | 0.14 | 0.46 | (-0.23, 0.51) | - | - | - |
In the univariate regression analysis, age, SCP-P, and DCP-P were found to significantly influence FMD (b=-0.38, b=0.36, and b=0.57, respectively). Parameters with a p-value lower than 0.1, plus age and sex, were included in the multivariate analysis. Due to the collinearity of DCP-P and SCP-P, it was decided to omit the latter from the multivariate analysis. In the final model, FMD was found to be dependent on the presence of HT (b=-0.4) and DCP-P (b=0.47) (Table 4).
4. DISCUSSION
In this study, in diabetic patients and healthy population, FMD was found to be significantly positively correlated to DCP. Our findings also indicated that FMD was influenced negatively by age and hypertension. Additionally, after adjusting for age and sex, FMD decreased with the presence of hypertension and lower perifoveal DCP. In our studied population, only CVI was significantly lower in diabetic patients than in healthy controls.
In 2023, Heiss et al. published FMD reference values for healthy individuals. Their meta-analysis reported a mean FMD of 6.4%, which was similar to the 6.13% obtained in our study population, containing DM patients and healthy controls. Age significantly influenced the reduction in FMD, which was consistent with our findings. A high heterogeneity was observed between studies despite similar methodologies being used [14]. The association of FMD with hypertension has been previously reported, as HT impairs vascular endothelial function, which influences the degree of arterial vasodilation [15, 16]. Importantly, we did not observe a significant difference in FMD values between the diabetic and healthy groups. It may be explained by the fact that patients in the DM group did not have diabetic complications, and their mean HbA1C level was not significantly elevated (7.6%), so the impairment of endothelial function may not have been significant. It is also possible that this relationship would change in a larger study population. FMD is increasingly recognized for its value not only as a measure of endothelial function but also as a potential predictor of cardiovascular events. Numerous studies have demons- trated that impaired FMD is associated with an elevated risk of atherosclerosis and coronary artery disease, suggesting its utility as a non-invasive tool for early detection of cardiovascular dysfunction [17-19]. The ability to track improvements in FMD following lifestyle or pharmacological interventions makes it a useful metric in both clinical and research settings. However, while FMD has predictive value, it is important to acknowledge that variability in measurement and patient-specific factors can affect its reliability as a universal predictor of cardiovascular outcomes [20-22].
CVI was significantly reduced in the DM group compared to the control group, both in the central and perifoveal regions. This finding aligned with previous studies reporting reduced CVI in patients with diabetes, even in the absence of visible changes in the eye fundus [23, 24]. We did not observe a difference in SCP and DCP, likely due to the small sample sizes, as previous studies have reported reductions in these parameters in diabetic patients [25, 26].
FMD was positively correlated with DCP-P in both study groups and with SCP-P and CVI-P, but only in the control group. This is the first study to observe these correlations, as the relationship among FMD and SCP, DCP, and CVI, to the best of our knowledge, has not been studied in a similar population. Additionally, in the regression analysis, DCP-P emerged as a sex and age-independent factor positively affecting FMD. It is worth mentioning that SCP-P also positively influenced FMD in the univariate analysis and exhibited collinearity with DCP-P. The higher vessel density in the retinal plexuses of patients with higher FMD indicated that endothelial status in the large circulation may be translated to micro- circulation. The relationship between FMD and densities of retinal plexuses has been previously studied in post-COVID-19 patients, but no correlation among macular SCP, DCP, and FMD has been found [27].
Diabetes results in impaired vascular endothelial function, especially in patients with long-term disease. It may be caused by a reduction in nitric oxide (NO) production [28]. NO is a crucial molecule for vasodilation, and its deficiency leads to a decrease in FMD. Lower NO concentration can cause the constriction of arterioles and a subsequent reduction in capillary density. The observed correlation among FMD, SCP, and CVI exclusively in the healthy cohort indicates that endothelial function is dysregulated in individuals with diabetes. The association of FMD with DCP may persist in the diabetic population as this vascular bed is less susceptible to ischemic changes than SCP [29].
Vascular endothelial status, as expressed by FMD, has been studied in various ophthalmic conditions. Bojic et al. demonstrated lower brachial FMD in patients with glaucoma compared to a healthy control group [30]. Yao et al. observed reduced FMD in patients with non-arteritic anterior ischemic optic neuropathy. However, this finding was not confirmed by Ma et al. when comparing with a hypertensive group [31, 32]. FMD was also found to be reduced in patients with branch retinal vein occlusion compared to both healthy and hypertensive groups [33]. Additionally, some authors have suggested a link between endothelial dysfunction and the pathophysiology of wet age-related macular degeneration [12].
The findings of our study suggest that eye micro- circulatory status is associated with vascular endothelial function in systemic circulation. However, further investigations involving larger cohorts are warranted to validate this relationship and identify parameters that can aid in predicting cardiovascular risk based on retinal and choroidal parameters.
Our study has several noteworthy limitations. Firstly, edge tracking software was not used in the calculation of FMD, which may have posed a risk of less accurate capture of maximum arterial diastole. Additionally, the study did not encompass a large cohort, potentially resulting in undetected relationships. Finally, despite statistical insignificance, the control group contained more females.
CONCLUSION
This is the first study to report the association of retinal capillary plexus density parameters with peripheral vascular endothelial function expressed by FMD in a healthy population and type 2 diabetes. Higher retinal vascular density, especially in the deep capillary plexus, was found to be associated with higher FMD. This relationship may, in the future, be used to identify additional ocular indicators of cardiovascular function.
AUTHORS’ CONTRIBUTION
A.M.: collected the data; J.P.-D.: carried out the data analysis or interpretation of the results; M.M.-H.: contributed to the concept or design of the study.
LIST OF ABBREVIATIONS
| FMD | = Flow-mediated Vasodilatation |
| CVI | = Choroidal Vascularity Index |
| OCTA | = Optical Coherence Tomography Angiography |
| NO | = Nitric Oxide |
| CT | = Choroidal Thickness |
| LA | = Luminal Area |
| TCA | = Total Choroidal Area |
| CVI | = Choroidal Vascularity Index |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Ethics Committee of Wroclaw Medical University, Poland (KB-510/2022).
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.


