All published articles of this journal are available on ScienceDirect.
Profiling of Retinopathy of Prematurity (ROP) Patterns at Various Gestational Ages in a Tertiary Care Institute in North India: A Retrospective Study
Abstract
Background
Retinopathy of prematurity (ROP) is a vasoproliferative retinal disorder that primarily affects preterm newborn children. The majority of ROP cases occur in developing countries because of socioeconomic conditions, large populations, and a lack of NICU care.
Methods
During the five years between 2019 and 2023, this retrospective study was conducted at a single tertiary care facility in North India. The patient's pupil was dilated using a diluted tropicamide and phenylephrine combination during the ROP screening process. Along with the neonatologist, oxygen and other life support, all aseptic precautions were followed throughout the assessment.
Results
Throughout the full-time frame, 700 preterm newborn infants who were screened had a mean birth weight (BW) of 1353 g ± SD of 430.48, and the mean GW was 28.66 ±SD of 1.12. Peripheral avascular (stage 0) and zone 3-stage 1 patients had the highest ROP cases. Similarly, 71.42%, 64.28%, 60.29%, 82.6%, 91%, 92.72%, 97.67%, 97.95%, 96.38%, 100%-&-100% of the ROP patients in GW26 to 36 matured without intervention in stage III compared to stage I ROP. Furthermore, analysis of our data revealed that 14.28%, 35.7%, 39.5%, 16%, 10%, 5%, 2%, 2%, and 3% of patients with ROP had GW26 to GW34, respectively. Of these, 14.28%, 25%, 22%, 10%, 7%, 2%, 2%, and 2% of ROP patients with 26–34 GW required intravitreal anti-VEGF injections in addition to LASER treatment, whereas 14.28%, 10.7%, 5.88%, and 1% of ROP patients with 26, 27, and 28 weeks of gestation, respectively, required only LASER treatment.
Conclusion
Hence, these findings suggest that the incidence of ROP is greater in the 27th and 28th GWs. These findings also suggest that as individuals grow from 31 to 36 GW, ROP regresses without intervention, indicating that premature births between 26 and 30 GW are more prone to ROP than pregnancies between 33 and 36 GW. These data imply that intravitreal anti-VEGF injections, in conjunction with laser treatment, are effective.
1. INTRODUCTION
Retinopathy of prematurity (ROP) is a potentially vision-threatening but treatable eye illness that primarily affects premature newborns weighing 1500 grams or less and/or those delivered before 34 weeks of gestation. This is one of the most prevalent causes of childhood vision loss, and if left untreated, it can lead to lifelong vision impairment and blindness. The prevalence of ROP ranges from 38% to 47% in various regions of India. The incidence of ROP is increasing due to a variety of factors, including an increase in the number of neonatal intensive care units, resulting in higher survival rates in preterm children. However, without controlled oxygen delivery, the incidence of ROP is high, particularly in rural regions. Furthermore, there is an increasing tendency to use artificial means of conception, such as IVF (in vitro fertilization), which results in numerous pregnancies and low birth weight and is another major risk factor for ROP [1-5].
ROP has been associated with several risk factors. It occurs only in premature children who delivered before 34 weeks and/or who weighed less than 1,500 grams at birth. Early exposure to high oxygen concentrations, multiple episodes of bradycardia, heart abnormalities, low vitamin E levels in the serum, uncontrolled convulsions, anemia, and blood transfusions are associated with other significant conditions, including genetic variation, among others [6, 7].
In general, evidence suggests that the lower the infant's birth weight, the greater the incidence of medical complications and the greater the likelihood of developing ROP because most of these medical conditions necessitate additional oxygen support for the infant, who is already premature and whose lungs and other organs are not fully functional [8]. India has the world's highest overall incidence of preterm babies. Although neonatal mortality remains high, it is steadily decreasing. The number of neonatal intensive care units (NICUs) in India is rapidly expanding, and NICUs are now accessible to people living in rural areas, resulting in a greater survival rate for preterm infants [1].
The main pathology is retinal blood vessel development in humans beginning at approximately 16 weeks of gestation, during which the vessel progresses from the optic disc to the periphery of the retina. The nasal retina is vascularized at up to 36 weeks of gestation, but the temporal retina takes approximately 40 weeks to complete vascularization because the distance between the optic disc and the orra serrata was greater on the temporal side of the eye than on the nasal side. As a result, it will take approximately 4 weeks for vessels to reach the Distal section of the temporal retina. Any impediment to this growth, such as an unregulated oxygen supply, will stop the vessel growth, leaving the retina avascular. This avascular retina becomes hypoxic, causing angiogenic factor (VEGF) to be released, resulting in the formation of aberrant and anarchic arteries throughout the retina [2, 3, 9].
ROP development is divided into two stages. After premature birth, vessel growth stops in the first phase. During this time, the vessels may be destroyed by a variety of factors, including lack of oxygen. Premature newborns exposed to greater oxygen tension after delivery had a decrease in the main hypoxia-triggered VEGF, leading to the development of retinal capillaries. This was one of the first occurrences in the history of ROP [10].
Owing to increasing oxygen demand, the peripheral retina, which is largely avascular or hypovascularized, becomes hypoxic in the second phase. As a result, abnormal development of retinal vessels occurred at that location. During this phase, excess hormones and growth factors are produced to provide adequate perfusion to increasingly hypoxic retinas. Specifically, VEGF and growth hormones such as IGF-1 are produced. These substances cause extracellular matrix proteins such as vitronectin, fibronectin, and fibrinogen to deposit sticky fibrils and drive endothelial cell proliferation, differentiation, and migration. These new vessels are chaotic and excessive, resulting in vitreous invasion, retinal traction, and hemorrhage. This key period of ROP most frequently occurs between 33 and 34 weeks after pregnancy. Traction on the retina results in tractional retinal detachment, which leads to blindness. In addition, there are genetic links. In a study of monozygotic and dizygotic preterm twins, secondary heritable variables were found to account for 70% of the variance in ROP incidence. Numerous gene variants, including those in the Wnt signaling pathway (FZD4, LRP5, and NDP), were discovered in small candidate gene investigations [2, 3, 10, 11].
There are many demographic profiles and ROP patterns available from North and South India, as well as internationally, but none of the data describe the pattern of ROP at different gestational stages. In light of this, the study's objective was to profile the retinopathy of prematurity (ROP) pattern at various gestational ages, ranging from 26 to 36 weeks, in a tertiary care hospital in North India. The newborn intensive care unit at the PGICH NOIDA is properly furnished. The Department of Ophthalmology receives a sizable number of preterm infants for ROP screening. Additionally, the department receives referrals from other sections of the Delhi NCR as well as from different regions of the state.
2. METHODS
2.1. Ethical Approval
The work was completed at the Department of Ophthalmology, Post-Graduate Institute of Child Health, Noida, with the necessary ethical clearance for consent waiver no. 2023-08-IM-45) as retrospective data from the Institutional Ethics Committee of the Post Graduate Institute of Child Health, Noida, India, approved by the Central Drugs Standard Control Organization (CDSCO), Registration No: ECR/1320/Inst/UP/2019 and Department of Health Research Registration No: EC/NEW/INST/2022/UP/0062, Government of India. The consent waiver was approved upon signing a privacy and confidentiality form with patient data identifiers as “Completely Anonymized (Subject cannot be identified)” in compliance with CDSCO and ICMR, Government of India rules, to participate in this study.
2.2. Patient Selection
All patients with a gestational age of less than 36 weeks and/or birth weight less than 1500 g were screened. Throughout the full timeframe from 2019 to 2023, 700 newborns were screened. Of these, 35 were excluded owing to incomplete information, 9 were excluded because the primary treatment was received elsewhere, and 13 were excluded due to a lack of follow-up. Finally, the data from 643 infants were examined. A bilateral fundus examination with scleral indentation was performed using an indirect ophthalmoscope to examine any avascular regions, abnormal vascular proliferation over the retina, or fibrovascular growth over the retina or into the vitreous cavity. The patients were asked whether the sickness was pre- or post-plus. Fundus examination was performed every 1–3 weeks, depending on the health of the retinal vessels. We followed the patients for up to 54 weeks, with increasing inspection intervals depending on the clinical results or all babies until their condition was resolved. To enable timely intervention, a monitoring system for neonates at risk of developing ROP has been created. In most cases, newborns under 36 weeks of gestation and/or with birth weights less than 1500 grams were subjected to the monitoring regimen. The first assessment was performed during the first two weeks of life, after which regular weekly examinations were conducted until it became clear that either none of the eyes would develop a disease that needed to be treated or that one or both would. Therapy was administered within 48 h because the illness could worsen quickly. We classified ROP according to international guidelines.
2.2.1. Stage 1
Mild disease, formation of demarcation line at the junction of vascular and avascular.
2.2.2. Stage 2
Moderate disease, elevation and increased width of a demarcation line to form a ridge.
2.2.3. Stage 3
Severely diseased, neovascularization typically extra-retinal and emanating from a ridge, with the exception of zone I, where stage 3 may be intra-renal.
2.2.5. Stage 5A
denotes a total retinal detachment with the optic nerve visible, an open funnel configuration to the detachment.
2.2.6. Stage 5B
Stage 5B denotes a total retinal detachment with the optic nerve NOT visible, a closed funnel.
2.2.7. Stage 5C
Includes the findings of Stage 5B along with anterior segment anomalies like a shallow anterior chamber, irido-lenticular adhesions and corneal opacity.
2.2.7.1. Aggressive-ROP- or A-ROP
Signs of A-ROP include rapid development of stage 3 with plus disease, extremely anomalous vasculature with shunting and vessel loops, and flat-appearing stage 3 without line or ridge demarcation.
2.2.7.2. Plus Disease
The appearance of dilation and tortuosity of retinal vessels, and “preplus disease” is defined as “abnormal vascular dilation and/or tortuosity insufficient for plus disease. Rather than peripheral vessel appearance, iris vessel engorgement, poor pupil dilation, peripheral retinal vessel engorgement, and vitreous haze have been used to define the level of plus disease, rather than peripheral vessel appearance, iris vessel engorgement, poor pupil dilation, peripheral retinal vessel engorgement, and vitreous haze.
2.8. Intervention
2.8.1. Laser Photocoagulation
We used the ETROP trial approach of laser photocoagulation to treat the pre-threshold sickness. Type 1 ROP requires treatment within 48 to 72 h, and type 2 ROP requires close observation and pre-threshold sick eyes.
(1) Zone 1, any stage with plus
(2) Zone 1, stage 3 without plus; and
(3) Zone 2, stage 2, or 3 with plus signs.
Treatment with type 1 ROP reduced adverse structural results from 15.6 to 9.0% and unfavorable visual acuity outcomes from 19.8 to 14.3% in the ETROP study.
2.8.2. Anti-vascular Endothelial Growth Factor (VEGF) Therapy
A dosage of 0.625 mg of bevacizumab in 0.025 ml of solution was administered in the operating theater under topical anesthesia; in cases of observed improvement, this dosage was repeated at intervals of two to three weeks. Retinal angiogenesis is induced by elevated levels of VEGF, and retinopathy of prematurity (ROP) results from aberrant neovascularization.
2.8.3. Surgery for Stage 4 and 5 ROP
Retinal detachment in patients with severe retinopathy of prematurity (ROP) was classified as stage 4 or 5. Surgical interventions included scleral buckling alone or vitrectomy with or without scleral buckling. While lensectomy may facilitate improved access to anterior tissues, it presents potential risks of increased glaucoma and refractive amblyopia. Surgical complications encompass glaucoma, strabismus, cataracts, and corneal opacities. The prognosis for stage 5 ROP is generally considered unfavorable.
2.8.4. Follow-up of ROP
Depending on the stage of ROP and clinical outcomes, follow-up examinations were conducted at intervals of less than one week, one to two weeks, and two to three weeks. Generally, clinical examinations were concluded based on age and clinical findings. The criteria for termination include zone 3 retinal vascularization without prior zone 1 or zone 2 ROP, complete retinal vascularization for 360 degrees at the ora serrata, a postmenstrual age of 54 weeks, and absence of prethreshold disease or ROP reversal. Regression denotes disease involution and resolution, which may occur spontaneously or as a consequence of treatment.
When retinal defects persist, this process is termed incomplete regression. Regression typically occurs more rapidly following anti-VEGF treatment (1-3 days) compared to laser photocoagulation alone (7-14 days). Clinical indicators of regression include diminished “plus” disease, reduced vascular dilatation and tortuosity, involution of the tunica vasculosa lentis, increased pupillary dilation, improved medium clarity, resolution of intraretinal hemorrhage, and attenuation and blanching of neovascular tissue. Subsequently, normal vascularization will proceed in the previously avascular peripheral retina.
The severity of reactivation of previously treated ROP ranged from a minor self-limiting demarcation line to stage 3+ illness. Skip areas after laser treatment can help with reactivation. However, reactivation frequently occurs sooner after laser treatment than after anti-VEGF medication.
2.9. Data Analysis
The data were analyzed using Microsoft Excel Statistical Software Package with the data analysis plugin for mean, mode, variance, standard deviation, and upper and lower confidence intervals with significance at a 95% confidence level. Nominal and categorical variables were described as proportions. Continuous variables were described as mean and standard deviation. The raw data from each patient were processed with Origin Graphical Data Analysis software, (https://www.originlab.com/origin) resulting in a chord diagram. The rationale for constructing a chord diagram was to provide a visual representation of the interconnections among the peripheral avascular space, zone 2 stage 1, zone 2 stage 2, zone 2 stage 3, zone 3 stage 1, and three-time points: 1ST ROP WK (%), 2ND ROP WK (%), and 3RD ROP WK (%). Chord diagrams offer the advantage of reducing visual complexity when depicting linkages between multiple entities using lines.
3. RESULTS
Throughout the study period, 700 neonates were screened. Of these, 35 patients were excluded due to incomplete information, 9 were excluded because primary treatment was received elsewhere, and 13 were excluded due to a lack of follow-up. Ultimately, the data of 643 infants were analyzed. The screened infants had a mean birth weight (BW) of 1353 g and a standard deviation (SD) of 430.48 g (range: 700 to 3200 g) (Table 1).
The range of gestation was 26 to 36 weeks, with a mean gestational age of 28.66 weeks and a standard deviation of 1.12 (Table 2).
The first week after NICU admission was the first follow-up week, called ROP stage I, and the second to third weeks, called ROP stages II and III, depending on the clinical findings and severity of the patients. All patients were followed up until the age of five years.
The data were further examined over a gestational period of 26–36 weeks. The findings were divided into nine categories: nil/ no ROP, peripheral Avascular, zone 2 stage 1, zone 2 stage 3, zone 3 stage 1, zone 3 stage 2, zone 3 stage 1, and retinal maturation.
| Birth Weight | Frequency | xm | f*xm | f*xm^2 | Mean | 1353.16 |
|---|---|---|---|---|---|---|
| 700-1100 | 185 | 900 | 166500 | 149850000 | Modal Class | 1100-1500 |
| 1100-1500 | 284 | 1300 | 369200 | 479960000 | Variance | 185314.85 |
| 1500-1900 | 87 | 1700 | 147900 | 251430000 | SD | 430.48 |
| 1900-2300 | 54 | 2100 | 113400 | 238140000 | Upper CI (95%) | 219.3846674 |
| 2300-2700 | 14 | 2500 | 35000 | 87500000 | Lower CI (95%) | -8.718000735 |
| 2700-3200 | 8 | 2900 | 23200 | 67280000 | - | - |
| - | 632 | - | 855200 | 1274160000 | - | - |
| Period of Gestation | Frequency | xm | f*xm | f*xm^2 | Mean | 28.66 |
|---|---|---|---|---|---|---|
| 26-27 | 42 | 26.5 | 1113 | 29494.5 | Modal Class | 27-29 |
| 28-29 | 137 | 27.5 | 3767.5 | 103606.25 | Variance | 1.25 |
| 30-31 | 219 | 28.5 | 6241.5 | 177882.75 | SD | 1.12 |
| 32-33 | 135 | 29.5 | 3982.5 | 117483.75 | Upper CI (95%) | 185.3157 |
| 34-35 | 91 | 30.5 | 2775.5 | 84652.75 | Lower CI (95%) | 23.01762 |
| 36-37 | 1 | 31.5 | 31.5 | 992.25 | - | - |
| - | 625 | - | 17911.5 | 514112.25 | - | - |
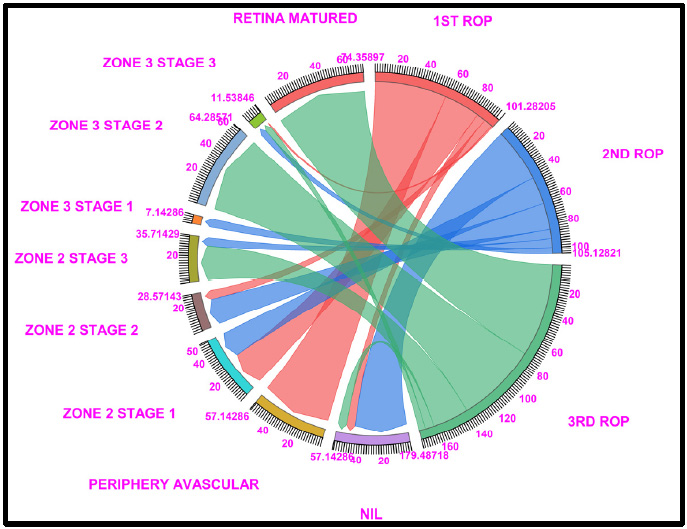
Chord diagram of the gestational period of 26: The graph shows the distributions of the numbers of patients with ROP, peripheral avascular, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 26 GW data show that, 57.14% of cases fallen under no ROP detected (7.14% in ROP 1st week, 42.85% of cases in ROP 2nd week and 7.14% in ROP 3rd week), 57.1% of peripheral avascular stages of cases fall under the first ROP and none of the cases shown in the second and third ROP, a total of 57.14% of cases in zone 2 stage 1 (28.57% in ROP 1st week and 21.42% of cases in ROP 2nd week), a total of 28.57% of cases in zone 2 stage 2 (7.14% in ROP 1st week and 21.42% of cases in ROP 2nd week), 35.71% of cases were represented on zone 2, stage 3 (7.14% in ROP 2nd week and 28.57% of cases in ROP 3rd week), 7.14% cases in zone 3, stage 1 and 64.28% of cases fall under Retina Matured in 3rd week of ROP checkup. Where Light Red, Blue, and Light Green represent the first, second, and third ROP week, respectively.
3.1. 26 Gestational Weeks
In total, 57.4% of the peripheral avascular tissue (stage 0), 28.57% of zone 2 stage 1, 7.14% of zone 2 stage 2, and no ROP occurred during the 1st ROP checkup. The percentage of patients in the no ROP category increased to 7-42% at the second ROP checkup compared with the 1st ROP checkup. Similarly, in Zone 2 stage 2, zone 2 stage 3 and zone 3 stage 1 increases to 21.42% and 7.14%, respectively. In the third ROP checkup, 28.5% of zone 2 stage 3 patients and 64.24% of ROP patients exhibited retinal maturity.
Of the 64.24% of retina-matured ROP patients, 71.42% matured without any intervention, whereas 14.28% of the patients were treated with intravitreal anti-VEGF injections + LASER or LASER (Fig. 1 and Table S1).
3.2. 27 Gestational Weeks
The 27 GW data showed that 42.8% of peripheral avascularity (stage 0), 17.85% of zone 2 stage 2, 14.28% of zone 3 stage 1, and 7.14% of no ROP, zone 2 stage 1, zone 2 stage 3, and 3.5% of zone 3 stage 2 cases were detected in the 1st ROP checkup. In the 2nd ROP checkup, the percentage of patients with no ROP increased to 46.4%, followed by 17% for zone 2 stage 1 and zone 2 stage 3 patients, 14% for zone 3 stage 1, and 10% for zone 2 stage 2 patients. Similarly, at the 3rd ROP checkup, 60.71% of the ROP patients had a mature retina, and 28.5% of the patients were diagnosed with zone 2 stage 3 ROP.
Of the 60.71% retina-matured ROP patients, 64.28% had matured without any intervention, 25% had been treated with intravitreal anti-VEGF injections + LASER,
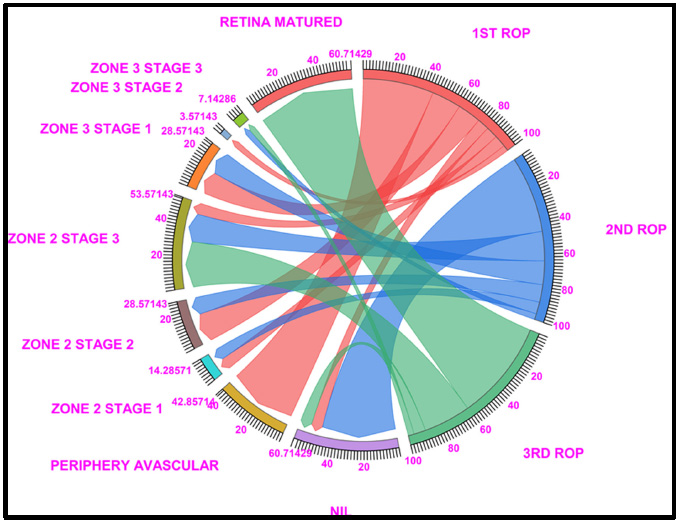
Chord diagram of the gestational period of 27: This graph displays the distribution of the number of patients with ROP, peripheral avascular, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 27 GW data show that, 60.71% of cases fallen under no ROP detected (7.14% in ROP 1st week, 46.42% of cases in ROP 2nd week and 7.14% in ROP 3rd week),,peripheral avascular stages (42.85% of cases fall under the first ROP and none of the cases shown in the second and third ROP), a total of 14.28% of cases in zone 2 stage 1 (7.14% in ROP 1st week and 7.14% of cases in ROP 2nd week), a total of 28.57% of cases in zone 2 stage 2 (17.85% in ROP 1st week and 10.71% of cases in ROP 2nd week), 53.57% of cases were represented on Zone 2, Stage 3 (7.14% in ROP 1st week, 17.85% in ROP 2nd week and 28.57% of cases in ROP 3rd week) 28.57% cases in Zone 3, Stage 1 (7.14% in ROP 1st week and 7.14% of cases in ROP 2nd week) and 60.71% of cases fall under Retina Matured in 3rd week of ROP checkup. Light Red, blue, and light green represent the first, second, and third ROP weeks, respectively.
and 10.7% had undergone LASER treatment (Fig. 2 and Table S2).
3.3. 28 Gestational Weeks
The 28GW data revealed that 32.35% of the peripheral avascular tissue (stage 0), 19.11% of the zone 3 stage 1, 11.76% of the no ROP, zone 2 stage 2, and 10.29% of the zone 3 stage 2 cases were detected at the 1st ROP checkup. In the 2nd ROP checkup, 17.64% of the patients with No ROP were diagnosed, followed by 20% of the zone 2 stage 2 patients, 11% of the zone 2 stage 3 patients, 29% of the zone 3 stage 1 patients, and 10% of the zone 3 stage 2 patients. Similarly, at the 3rd ROP checkup, 54.41% of the ROP patients had retinal maturation, and 17.6% of the patients were diagnosed with zone 2 stage 3 ROP.
Out of 54.41% of the retina-matured ROP patients, 60.29% had matured without any intervention, 22% had been treated with intravitreal anti-VEGF injections + LASER, 11.7% had been treated with LASER, and 5.88% had been treated with LASER alone (Fig. 3 and Table S3).
3.4. 29 Gestational Weeks
The 29 GW data showed that 37.68% of zone 3 stage 1 patients had 21.73% peripheral avascular tissue (stage 0), 13.04% of zone 2 stage 1 patients, 10.14% of zone 3 stage 2 patients, and 8.69% of patients with no ROP were detected at the 1st ROP checkup. In the 2nd ROP checkup, 21.73% of the no ROP patients had ROP, 46.37% had stage 1, and 11% had stage 3. Similarly, at the 3rd ROP checkup, 71.01% of the ROP patients had a mature retina, and 10.14% of the patients were diagnosed with zone 3 stage 1 ROP.
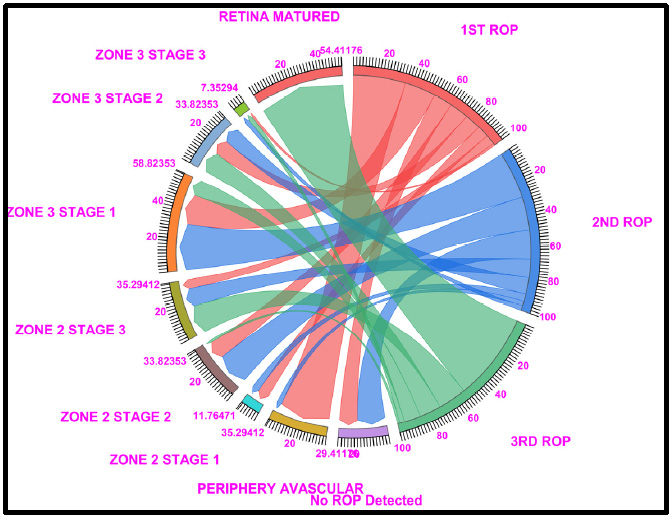
Chord diagram of the gestational period of 28: This graph displays the distribution of the number of cases with ROP, peripheral avascular, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina matured ROP in the first, second, and third first ROP weeks. the 28 GW data show that 29.41% of cases fallen under no ROP detected (11.76% of cases in ROP 2nd week and 17.64% in ROP 3rd week), 35.29% of peripheral avascular stages (32.35% of cases fall under the first ROP and 2.94% of cases in ROP 2nd week), a total of 11.76% of cases in zone 2 stage 1 (7.35% in ROP 1st week and 4.4% of cases in ROP 2nd week), a total of 33.82% of cases in zone 2 stage 2 (11.76% in ROP 1st week and 20.58% of cases in ROP 2nd week and 1.4% in 3rd ROP week), 35.29% of cases were represented on Zone 2, Stage 3 (5.8% in ROP 1st week, 11.76% in ROP 2nd week and 17.64% of cases in ROP 3rd week), 58.82% cases in Zone 3, Stage 1 (19.11% in ROP 1st week and 29.41% of cases in ROP 2nd week and 10.29% of cases in ROP 3rd week), 33.82% cases in Zone 3, Stage 2 (10.29% in ROP 1st week and 20.29% of cases in ROP 2nd week and 13.23% of cases in ROP 3rd week) and 54.41% of cases fall under Retina Matured in 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
Of the 71.01% of the retina-matured ROP patients, 82.6% had matured without any intervention, 10% were treated with intra-vitreal anti-VEGF injections + LASER, 4% were treated with LASER, and 2% were treated with LASER alone (Fig. 4 and Table S4).
3.5. 30 Gestational Weeks
The 30 GW data showed that 60.55% of the patients had no ROP and 27.52% of the patients had zone 3 stage 1 ROP according to the 1st ROP checkup. In the 2nd ROP checkup, 61.46% of the patients had no ROP, whereas 29.35% had stage 1 disease. Similarly, at the 3rd ROP checkup, 88.07% of the ROP patients had a mature retina, and 4.5% of the patients had zone 3 stage 3 ROP.
Among 88.07% of retina-matured ROP patients, 91% had matured without any intervention, 7% had been treated with intravitreal anti-VEGF injections + LASER, 2% had been treated with AVGEF, and only 1% had been treated with LASER alone (Fig. 5 and Table S5).
3.6. 31 Gestational Weeks
The 31 GW data showed that 73.63% of the patients had no ROP and 16.51% of the patients had zone 3 stage 1 ROP according to the 1st ROP checkup. In the 2nd ROP checkup, 62.72% of the patients did not have ROP, followed by 28.18% of the patients in zone 3 stage 1. Similarly, in the 3rd ROP checkup, 91.81% of the ROP patients were retinal mature, and 2.7% of the patients were diagnosed with zone 3 stage 2 or zone 3 stage 3.
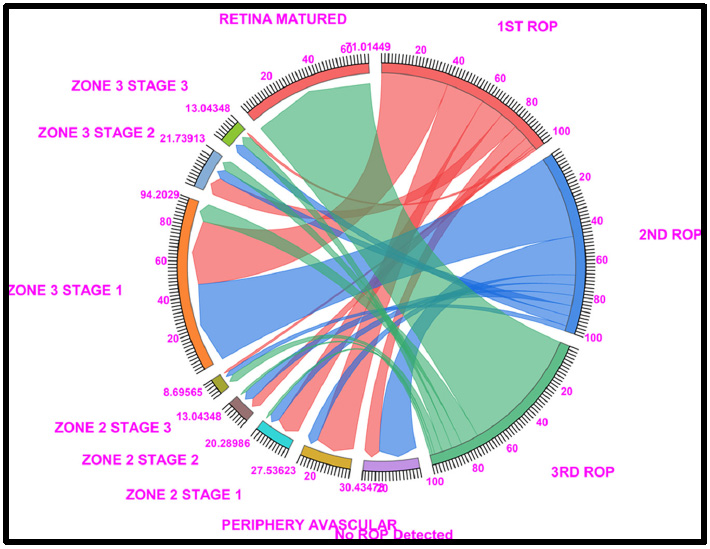
Chord diagram of the gestational period of 29: This graph displays the distributions of the numbers of patients with ROP, peripheral avascular ROP, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 29 GW data show that, 30.43% of cases fallen under no ROP detected (8.66% of cases in ROP 1st week and 21.73% in ROP 2nd week), 27.53% of peripheral avascular stages (21.73% of cases fall under the first ROP and 5.79% of cases in ROP 2nd week), a total of 20.28% of cases in zone 2 stage 1 (13.04% in ROP 1st week and 5.790% of cases in ROP 2nd week), a total of 20.28% of cases in zone 2 stage 2 (5.7% in ROP 1st week and ROP 2nd week each and 1.4% in 3rd ROP week), 94.20% cases in Zone 3, Stage 1 (37.68% in ROP 1st week and 46.36% of cases in ROP 2nd week and 10.14% of cases in ROP 3rd week), 21.73% cases in Zone 3, Stage 2 (10.14% in ROP 1st week and 5.7% of cases in ROP 2nd week and 5.7% of cases in ROP 3rd week) and 71.01% of cases fall under Retina Matured in 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
Among 91.81% of retina-matured ROP patients, 92.72% had matured without any intervention, 2% had been treated with intravitreal anti-VEGF injections + LASER, 1% had been treated with AVGEF, and 2% had been treated with only LASER injections + LASER, 1% had been treated with AVGEF, and 2% had been treated with only LASER (Fig. 6 and Table S6).
3.7. 32 Gestational Weeks
The 32 GW data showed that 76.74% of the patients had no ROP and 16.27% of the patients had Zone 3 Stage 1 ROP according to the 1st ROP checkup. In the 2nd ROP checkup, 72.09% of the patients were not ROP and 24.41% were zone 3 stage 1. Similarly, at the 3rd ROP checkup in 95 patients, 34% of the ROP patients had retinal maturation, 2.0% had zone 3 stage 2, and 2.0% had zone 3 stage 3.
Of the 91.81% of retina-matured ROP cases, 97.67% of cases were matured without any intervention, whereas 1% of cases were treated with intravitreal anti-VEGF injections, and only 1% were treated with only LASER treatment (Fig. 7 and Table S7).
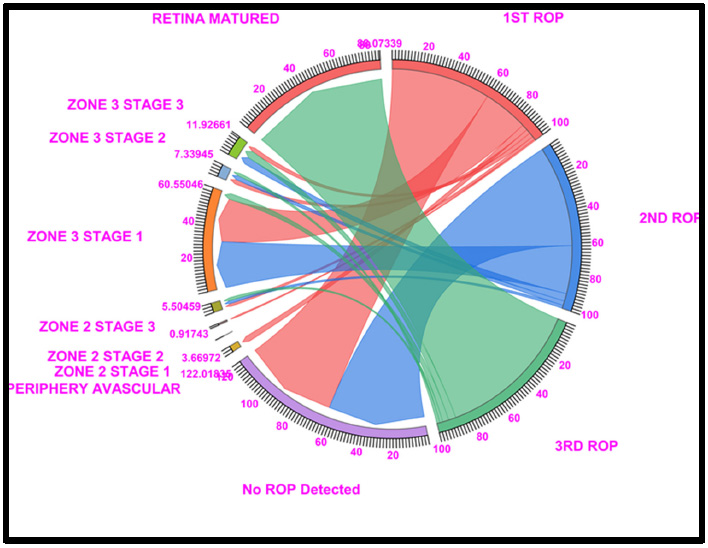
Chord diagram of the gestational period of 30: The graph shows the distributions of the numbers of patients with ROP, peripheral avascular ROP, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 30GW data show that, Under no ROP, 60.55% of cases in ROP 1st week and 61.46% in ROP 2nd week, dramatically, 3.6% of peripheral avascular stages; a total of 60.55% of cases in zone 3 stage 1 (27.52% in ROP 1st week and 29.35% in ROP 2nd week) and 88.07% of cases fell under Retina Matured in the 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
3.8. 33 Gestational Weeks
The 33 GW data showed that 81.63% of the no ROP patients and 14.28% of the zone 3 stage 1 patients were detected at the 1st ROP checkup. In the 2nd ROP checkup, 81.63% of the patients did not have ROP, followed by 14.28% of the patients in zone 3 stage 1. Similarly, during the 3rd ROP checkup, 97.95% of the ROP patients were retina-mature, and 2.0% of the patients had zone 2 stage 3 ROP.
Among the 97.95% of retina-matured ROP patients, 97.95% had matured without any intervention, whereas 2% were treated with intravitreal anti-VEGF injections and LASER treatment (Fig. 8 and Table S8).
3.9. 34 Gestational Weeks
The 34 GW data showed that 71.54% of patients had no ROP and 10.84% of zone 3 stage 1 patients had no ROP according to the 1st ROP checkup. In the 2nd ROP checkup, 86.74% of the patients had no ROP and 9.63% had zone 3 stage 1 ROP. Similarly, at the 3rd ROP checkup, 96.38% of the ROP patients had mature retinas.
Among the 96.38% of retina-matured ROP patients, 96.38% had matured without any intervention, whereas 2% were treated with intravitreal anti-VEGF injections and LASER treatment (Fig. 9 and Table S9).
3.10. 35 Gestational Weeks
The 35 GW data showed that 87.5% of the patients had no ROP and 12.5% of the patients had zone 3 stage 1 ROP according to the 1st ROP checkup. In the 2nd ROP checkup, 87.5% of the patients had no ROP, 12.5% had zone 3 stage 1, and 2 had zone 3 stage 2. Similarly, at the 3rd ROP checkup, 87.5% of the ROP patients had a mature retina and 12.5% had zone 3 stage 1 ROP.
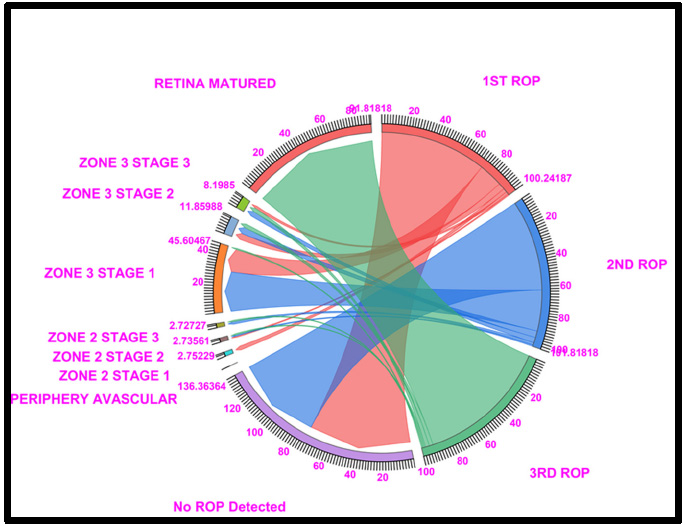
Chord diagram of the gestational period of 31: This graph displays the distribution of the number of patients with ROP, peripheral avascular, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 31GW data show that, No ROP was detected in 73.63% of ROP cases in the 1st week and 62.72% in the 2nd week, a total of ROP, 45.60% of cases in zone 3 stage 1 (16.51% in ROP 1st week and 28.18% of cases in ROP 2nd week), and 91.81% of cases fell under Retina Matured in the 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
Of the 87.5% of retina-matured ROP patients, 100% had matured without any intervention, and of 87.5% of the retina-matured ROP patients, 100% had matured without any intervention (Fig. 10 and Table S10).
3.11. 36 Gestational Weeks
The 36 GW data showed that 100% of zone 3 stage 1 was detected during the 1st ROP checkup. As for the 2nd ROP checkup, 100% of the patients had zone 3 stage 1 ROP. Similarly, at the 3rd ROP checkup, 100% of the ROP patients had mature retinas, and all 100% of the patients had matured without any intervention (Fig. 11 and Table S11).
The regression data revealed a strong positive correlation between ROP regressed/not ROP and intravitreal anti-VEGF injections + LASER cases (r = 0.98, p > 8.75E-08, and r square = 0.96) and that the overall variation between predicted ROP regressed/not ROP and intravitreal anti-VEGF injections + LASER cases was very high (96%) (Table S12a-c). We further analyzed the ROP regressed/no ROP data with the predicted ROP regressed/no ROP for intravitreal anti-VEGF injections + LASER cases and found that the variation was very low throughout the gestation period (Table 3; Fig. S1).
The regression data revealed a strong positive correlation between ROP regressed/not ROP and intravitreal anti-VEGF injection (r = 0.94, p > 1.53E-05, and r square = 0.88) and that the overall variation between predicted ROP regressed/not ROP and intravitreal anti-VEGF injection was very high (88%) (Table S13a-c). We further analyzed the ROP regressed/no ROP data and the predicted ROP regressed/no ROP data for patients receiving intravitreal anti-VEGF injections and found that the variation was very low throughout the gestation period (Table 4; Fig. S2).
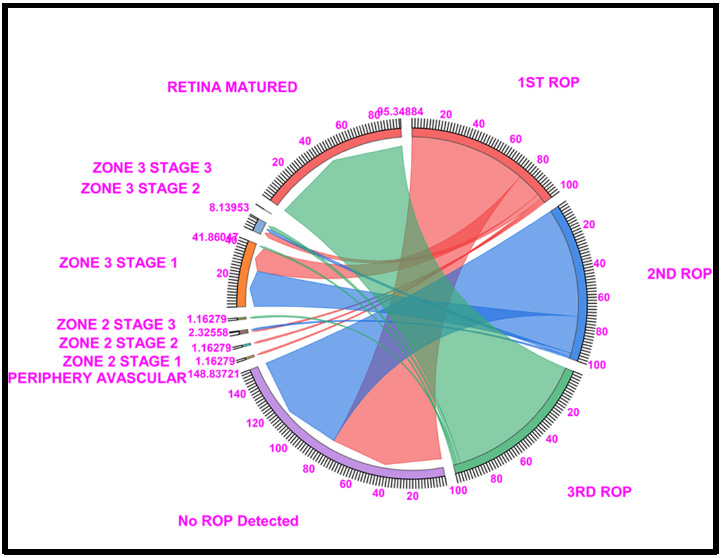
Chord diagram of the gestational period of 32: This graph displays the distribution of the number of patients with ROP, peripheral avascular, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 32 GW data showed that, Under no ROP detection, 76.74% of cases in ROP 1st week and 72.09% in ROP 2nd week, a total of 41.86% of cases in zone 3 stage 1 (16.26% in ROP 1st week and 24.41% in ROP 2nd week), and 95.34% of cases fell under Retina Matured in the 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
The regression data revealed that there was a low positive correlation between ROP regressed/no ROP and LASER cases (r = 0.43, p > 4.18E-08, and r square = 0.18) and that the overall variation between the predicted ROP regressed/NO ROP and LASER cases was very low (18%) (Table S14a-c). We further analyzed the ROP regressed/no ROP data with the predicted ROP regressed/no ROP data for patients with LASER and found that the variation was very large throughout the gestation period (Table 5 and Fig. S3).
4. DISCUSSION
The study was conducted at a single tertiary care institute in North India over a four-year period between 2019 and 2023. Throughout the study period, 700 preterm newborns were screened. A total of 643 preterm babies underwent analysis, although there was covid pandemic during this period. The infants who were screened had a mean birth weight (BW) of 1353 g and an average birth weight (SD) of 430.48 (ranging from 700–3200 g). With an SD of 1.12, the mean gestational duration was 28.66 weeks, with a range of 26 to 36 weeks. Similar statistics have been reported by Tekchandani et al. [12] when analysis of data from 2595 of the 3697 infants revealed a 32.3% overall incidence of ROP. The mean BW of children with ROP in the present study was 1353 g, which is greater than the 1277 g found in a 2021 study from India [12] as well as studies from Hong Kong [13], Taiwan [14], and Brazil [15]. Similarly, at 28.66 weeks, the mean gestational age in the present study was greater than that worldwide [12-15]. Hence, according to a previous Western study, ROP is more prevalent in newborns with higher birth weights and longer average gestation weeks than in neonates with lower birth weights.
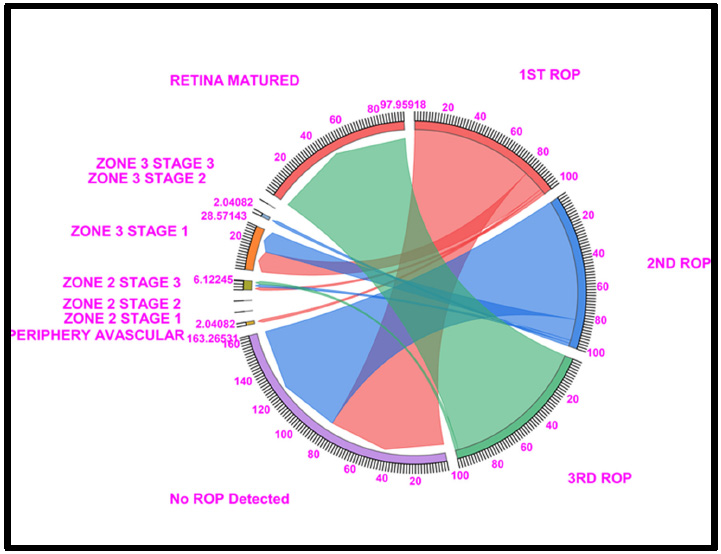
Chord diagram of the gestational period of 33: This graph displays the distribution of the number of patients with ROP, peripheral avascular, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 33 GW data show that Under no ROP detection, 81.63% of cases in ROP 1st week and 81.63% in ROP 2nd week, a total of 28.57% of cases in zone 3 stage 1 (14.28% in ROP 1st week and 14.28% in ROP 2nd week) and 97.95% of cases fell under Retina Matured in the 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
| Observation | Predicted ROP REGRESSED/NO ROP | Residuals | Standard Residuals |
|---|---|---|---|
| 1 | 76.21141184 | -4.78284 | -1.69185 |
| 2 | 58.98263051 | 5.303084 | 1.875875 |
| 3 | 63.7120999 | -3.41798 | -1.20905 |
| 4 | 82.86987805 | -0.26118 | -0.09239 |
| 5 | 88.85638896 | 1.969299 | 0.696606 |
| 6 | 94.7976123 | -2.07034 | -0.73235 |
| 7 | 99.18312027 | -1.5087 | -0.53368 |
| 8 | 95.90144764 | 2.057736 | 0.727889 |
| 9 | 95.30837427 | 1.077168 | 0.38103 |
| 10 | 99.18312027 | 0.81688 | 0.288957 |
| 11 | 99.18312027 | 0.81688 | 0.288957 |
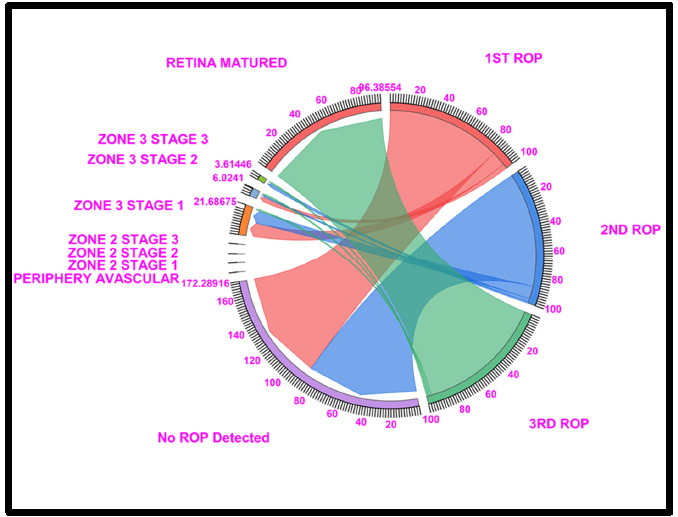
Chord diagram of the gestational period of 34: The graph shows the distributions of the numbers of patients with ROP, peripheral avascular ROP, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 34 GW data showed that In the absence of ROP, 85.54% of ROP cases in the 1st week and 86.74% in the 2nd week, and 96.37% of cases fell under Retina Matured in 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
| Observation | Predicted ROP REGRESSED/NO ROP | Residuals | Standard Residuals |
|---|---|---|---|
| 1 | 60.66074987 | 10.76782 | 2.154187 |
| 2 | 69.79925107 | -5.51354 | -1.10303 |
| 3 | 67.1114566 | -6.81734 | -1.36386 |
| 4 | 86.08962277 | -3.48093 | -0.69639 |
| 5 | 94.86724978 | -4.04156 | -0.80855 |
| 6 | 92.56242679 | 0.164846 | 0.032979 |
| 7 | 94.2394287 | 3.43499 | 0.687197 |
| 8 | 97.21475467 | 0.744429 | 0.148929 |
| 9 | 97.21475467 | -0.82921 | -0.16589 |
| 10 | 97.21475467 | 2.785245 | 0.55721 |
| 11 | 97.21475467 | 2.785245 | 0.55721 |
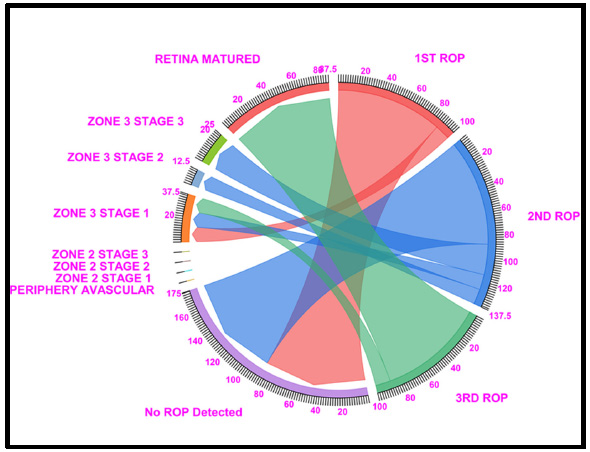
Chord diagram of the gestational period of 35: The graph shows the distributions of the numbers of patients with ROP, peripheral avascular ROP, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 35 GW data showed that Under no ROP detection, 87.5% of cases in the ROP 1st week and 87.5% in the 2nd week, and 87.5% of cases fell under Retina Matured in the 3rd week of ROP checkup. Where Light Red represents the first ROP week, Blue represents the second ROP week, and Light green represents the third ROP cases.
| Observation | Predicted ROP REGRESSED/NO ROP | Residuals | Standard Residuals |
|---|---|---|---|
| 1 | 91.73666 | -20.3081 | -1.52205 |
| 2 | 91.73666 | -27.4509 | -2.0574 |
| 3 | 71.17584 | -10.8817 | -0.81557 |
| 4 | 81.60524 | 1.003453 | 0.075207 |
| 5 | 85.32319 | 5.502495 | 0.412402 |
| 6 | 82.20392 | 10.52336 | 0.788706 |
| 7 | 87.67231 | 10.00211 | 0.749639 |
| 8 | 91.73666 | 6.222526 | 0.466367 |
| 9 | 87.52541 | 8.860135 | 0.664051 |
| 10 | 91.73666 | 8.263342 | 0.619322 |
| 11 | 91.73666 | 8.263342 | 0.619322 |
The majority of ROP disease presentations occurred in Peripheral Avascular and zone 3 stage 1, which was comparable to the findings of other studies, such as Tekchandani et al., 2021, in which only zone 2 cases were found [12]; Hungi et al., 2012, in which aggressive posterior ROP was discovered in zone 1 [16]; and Charan et al., 1995, in which stage 1 and stage 2 cases were both discovered [17]. Yehiam et al. (2023) reported both Group 1 and Group 2 instances in New Zealand from January 2013 to December 2017 [18]. Rutnin and Schepens et al. studied incomplete peripheral retinal vascularization in both adults and children [19, 20]. Ho et al. reported two cases of zone 3 stage 2 ROP [21]. Hence, these studies indicate that the prevalence of ROP in this region of the world is predominantly peripheral Avascular and zone 3, with the majority occurring at 28.66 weeks of gestation.
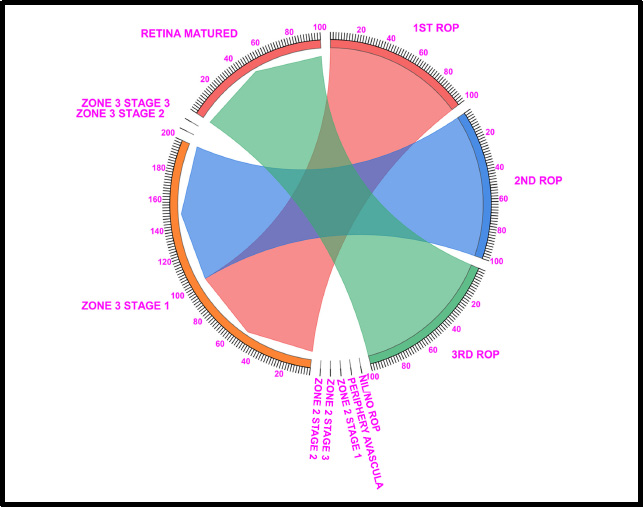
Chord diagram of the gestational period of 36: This graph displays the distribution of the number of patients with ROP, peripheral avascular, zone 2 stage 1, zone 2 stage 2, zone 3 stage 1, and retina mature ROP in the first, second, and third first ROP weeks. The 36GW data show that 100% of Zone 3 Stage 1 was detected in the 1st ROP checkup. As for the 2nd ROP checkup, 100% of the patients had Zone 3 Stage 1 ROP. Similarly, at the 3rd ROP checkup, 100% of the ROP patients had mature retinas, and all 100% of the patients had matured without any intervention.
Further analysis of the data from the range of 26 to 36 weeks of gestation revealed that 14.28% of the patients had ROP at 26 weeks of gestation, 35.7% had ROP at 27 weeks of gestation, 39.5% had ROP at 28 weeks of gestation, 16% had ROP at 29 weeks of gestation, 10% had ROP at 30 weeks of gestation, 5% had ROP at 31 weeks of gestation, 2% had ROP at 32 weeks of gestation, and 3% had ROP at 33 and 34 weeks of gestation; interestingly, all the ROP were mature without intervention at 35 and 36 weeks of gestation. The incidence of ROP varies by location in India, ranging from 38% to 47% in other parts of the country [12, 16, 17], and similar trends have been observed in other parts of the world, including 16% in Hong Kong [13], 37% in Taiwan [14], and 25.5% in Brazil [15]. These data indicate that the prevalence of ROP is greater during birth at 28th weeks of gestation and that the number of ROP cases decreases with increasing gestational age. A similar trend was observed by Ugurlu et al. in 2021, who concluded that newborns with a gestational age of 28 weeks accounted for 3.3% of all births and that the ROP rate was substantially greater than that of infants with older gestational ages (P 0.001) [22]. This could be related to the necessity for higher oxygen delivery during the 27th and 28th weeks of gestation, as opposed to the 35th and 36th weeks of gestation, implying that an uncontrolled oxygen supply in newborn critical care units may increase the incidence of ROP. However, there is an increasing trend toward artificial methods of conception, such as IVF, which could be another reason for the high prevalence of ROP [1].
Furthermore, detailed analysis revealed that compared to those with stage I ROP, 71.42%, 64.28%, 60.29%, 82.6%, 91%, 92.72%, 97.67%, 97.95%, 96.38%, and 100% of ROP patients at 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, and 36 gestational weeks, respectively, matured without any intervention in stage III ROP. These data indicate that as gestational age increases from 31 to 36 weeks, ROP regresses without any intervention, indicating that premature birth at 26 to 30 weeks is more susceptible to ROP than gestation at 33 to 36 weeks. However, studies report that babies with a gestational age ≤35 weeks and a gestational weight of 2000 grams are at a greater risk of developing ROP [22, 23]. Preterm birth under 28 weeks is the main cause of ROP in developed nations, although severe ROP can occur up to 34 weeks in developing countries [24-27].
These findings indicate that children between 26 and 30 weeks of gestation may develop advanced ROP that requires therapy. In contrast, Bas et al. (2024) found that newborns with higher gestational ages and weights may develop advanced ROP that requires treatment [28].
Similar trends were observed in the intravitreal anti-VEGF agent + LASER treatment group, in which 14.28%, 25%, 22%, 10%, 7%, 2%, 2% and 2% of the ROP patients were at 26 weeks, 27 weeks, 28 weeks, 29 weeks, 30 weeks, 31 weeks, 33 weeks and 34 weeks, respectively, needed AVEGF + LASER treatment. However, 14.28%, 10.7%, and 5.88% of ROP patients at 26, 27, and 28 weeks of gestation, respectively, and 1-2% of those at 29, 30, 31, 32, and 33 weeks of gestation needed LASER, respectively. These findings suggest that intravitreal anti-VEGF injections combined with laser treatment are more effective than laser treatment alone. Studies have reported that the combination of intravitreal anti-VEGF agents and minimally invasive laser therapy improves visual acuity (VA), relieves diabetic macular edema (DME) and may reduce the number of anti-VEGF agent injections required [29]. However, in ROP patients, anti-VEGF drugs are as effective as laser treatment and are safer than laser therapy for type 1 ROP [30]; however, Bezman et al., in 2023, reported that anti-VEGF agents are increasingly used in the treatment of ROP patients in recent time [31].
The ROP REGRESSED/NO ROP data were statistically analyzed in comparison to the predicted ROP REGRESSED/NO ROP data for patients treated with intravitreal anti-VEGF injections + LASER or intravitreal anti-VEGF injections, and the variation was found to be very low in all gestation period patients, indicating that intravitreal anti-VEGF injections + LASER and intravitreal anti-VEGF injection treatment was more suitable than LASER alone. Sen et al. (2021) endorsed the study and reported that combination therapy is an effective and safe treatment method for Type I ROP and Aggressive Posterior ROP [32]. However, Lingue et al., (2022) reported, in contrast to laser therapy, anti-VEGF drugs as primary therapies provide prospective benefits for eyes with zone I type 1 ROP [30]. Laser photocoagulation and anti-VEGF agent therapy were equally effective for treating eyes with zone II type 1 ROP; however, the rate of reactivation with laser therapy was much lower than that with anti-VEGF medications. Compared to anti-VEGF medication, laser treatment increases the tendency toward myopia [30]. According to Tran et al., anti-vascular endothelial growth factor (anti-VEGF) medication is increasingly being used off-label to treat ROP, but laser photocoagulation remains the gold standard for treating threshold and pre-threshold type 1 ROP [33].
CONCLUSION
These findings also suggest that the incidence of ROP is greater at the 27th and 28th weeks of gestation and that the number of ROP patients decreases with increasing gestational age. These findings suggest that as gestational age increases from 31 to 36 weeks, ROP regresses without intervention, indicating that premature births between 26 and 30 weeks are more prone to ROP than pregnancies between 33 and 36 weeks. These data imply that intravitreal anti-VEGF injections in conjunction with laser treatment are more effective than LASER treatment or intravitreal anti-VEGF injections alone.
AUTHORS’ CONTRIBUTIONS
V.S. and D.K.S.: Conceptualization of the experiments was provided; material preparation and experiment were contributed by; V.S.N.C., D.J. and S.M.:, data curation was provided by D.K.S.:, formal analysis was presented by D.K.S., V.S., N.C., D.J. and S.M. contributed to the investigation, methodology was adopted by; V.S., N.C., D.J. and S.M., D.K.S. was involved in writing - original draft and writing - review & editing. All the authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
| ROP | = Retinopathy of Prematurity |
| GW | = Gestational Weeks |
| VEGF | = Vascular Endothelial Growth Factor |
| NICU | = Neonatal Intensive Care Unit |
| BW | = Birth Weight |
| DME | = Diabetic Macular Edema |
| VA | = Visual Acuity |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The work was approved by the Institutional Ethics Committee of the Post Graduate Institute of Child Health, Noida, India, IEC protocol no. 2023-10-IM-45, approved by the Central Drugs Standard Control Organization (CDSCO), Registration No: ECR/1320/Inst/UP/2019 and the Department of Health Research Registration No: EC/NEW/INST/2022/UP/0062, Government of India.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
The consent waiver was approved upon signing a privacy and confidentiality form with patient data identifiers as “Completely Anonymized (Subject cannot be identified)” in compliance with CDSCO and ICMR, Government of India rules, to participate in this study.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available as Supplementary materials in the article. There is no such date created to be deposited in the databases.
FUNDING
This study was funded by Intramural: Postgraduate Institute of Child Health, India. Funder ID. IEC protocol no. 2023-10-IM-45.
ACKNOWLEDGEMENTS
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. We are very grateful to the Director Post Graduate Institute of Child Health, Noida, India, for facilitating the work.


