All published articles of this journal are available on ScienceDirect.
Effect of Systemic Blood Pressure and Follicular Stimulating Hormone on Retinal Blood Flow in Women: A Cross-sectional Study Using Optical Coherence Tomography Angiography (OCT-A)
Abstract
Purpose
This study aimed to investigate the influence of age-induced changes in Follicle-stimulating Hormone (FSH) and systemic Blood Pressure (BP) levels on the retinal vasculature in healthy women.
Methods
Optical Coherence Tomography Angiography (OCTA) was used to measure the Capillary Density Index (CDI%) of the retinal Superficial Vascular Plexus (SVP) of 124 eyes of healthy women aged 20 to 69 years. Findings were analyzed based on age, serum FSH levels, systemic (BP), and Body Mass Index (BMI).
Results
The average CDI% for all sections of the SVP from pooled data was 47.9±5.6%. The average CDI% for the four quadrants was 48.4±6.6%, 48.6±5.6%, 48.03±4.9%, and 46.4±4.6% for the superior, inferior, temporal, and nasal, respectively. A significant association between age and FSH levels was found in women aged 40-69 years (Spearman correlation coefficient (r) =0.8, p<0.0001); however, it did not influence the CDI% values, BP and BMI in our sample.
Conclusion
The present data provide evidence that healthy women continue life to older age without any deleterious effect on ocular hemodynamics despite the cessation of gonadal hormones.
1. INTRODUCTION
Retinal blood flow is one of the ophthalmic research areas that recently received attention for its implication in the pathogenesis of ocular diseases such as glaucoma and Age-related Macular Diseases (AMD) [1-3]. Several vasoactive mediators have been implicated in the pathogenesis of impaired blood flow and the induction of ocular disorders. For example, dysregulation of endothelial-derived factors, most importantly nitric oxide as a vasodilator and endothelin-1, a vasoconstrictor, contributes to ischemic events in the retina. The long-term result is the death of retinal ganglion cells and damage to the optic nerve, which constitutes the hallmark of glaucoma [1, 2]. Comparably, sex hormones have been found to play a vital role in the health and disease of ocular tissues [4]. These hormones have been recognized to influence the ocular blood flow, regulate intraocular pressure, and exert neuroprotective effects [4]. However, gender differences are evident when ocular diseases are discussed in relation to sex hormones, as women are more affected by hormonal changes rather than men. One reason is that the fluctuation of sex hormone levels, particularly estrogen and progesterone, occurs during the female life cycle, especially during menopause.
This critical stage for women is associated with overwhelming symptoms and may blossom to the onset of systemic and ocular diseases. The reduction in estrogen levels in postmenopausal women has been associated with changes in the horizontal curvature of the cornea, increased eye dryness, dysregulation of intraocular pressure, increased vascular resistance, and reduced blood flow to the ocular tissues [5-10].Evidence of estrogen protection has been obtained from studies in women undergoing bilateral oophorectomy before the age of 43, as the cessation of this hormone leads to early onset glaucoma [11]. In the same context, premature menopause has been associated with a higher prevalence of glaucoma [12]. Earlier clinical studies have revealed that Hormone RePlacement Therapy (HRT) reduced the IOP in postmenopausal women [13, 14], as estrogen replenishment regulates aqueous humor drainage, reduces intraocular pressure (IOP) [7], and increases ocular blood flow [15].
This growing evidence of estrogen-dominant beneficence in women directed the aim of this research to investigate the status of ocular blood flow in healthy women of different age groups using Optical Coherence Tomography Angiography (OCTA). This non-invasive device enables in vivo, cross-sectional imaging of the retina. This allows clinicians to assess the retinal vasculature and monitor the progression of conditions such as diabetic retinopathy and glaucoma. The principle of OCTA is based on the motion of particles, such as the red blood cells within the retinal vessels. The retinal layers can be viewed and segmented as Superficial Vascular Plexus (SVP) and deep plexus [16]. The SVP includes vasculature from the nerve fiber layer and ganglion cell complex, while the deep plexus contains the intermediate and deep inner retinal vasculature [16]. The current examination focuses on the SVP segment primarily affected in optic nerve diseases such as glaucoma. We hypothesized that hormonal deficiency affects the retinal vasculature in older women and hence induces a reduction in retinal blood flow. To achieve the goal of this research, healthy women of reproductive age, perimenopausal, and postmenopausal stages were recruited, and the capillary density of the SVP was assessed among different age groups using OCTA.
2. METHODOLOGY
2.1. Study Design and Participants
A total of 124 women visiting the ophthalmic clinic at Alderiyah Hospital, Riyadh, for routine ocular examination were enrolled in this cross-sectional study. Informed consent was obtained from each participant after explaining the nature of the research and before initiating data collection following the Helsinki Declaration. The study design was approved by the Ethical Committee of King Saud University (Research Project No. E-23-7606).
A detailed medical and ophthalmic history was obtained through the medical files and confirmed by interviewing the participant and asking questions about the systemic and ocular condition and current fertility status. Patients were excluded if they had a history of ocular disease, such as glaucoma, diabetic retinopathy, retinitis pigmentosa, retinal vein occlusion, and any retinal conditions. Chronic systemic diseases include anemia, cardiovascular, renal, or hepatic disorders, hypertension, and dyslipidemia. In addition, smokers, pregnant women, or those with a history of irregular menstruation, treatment with oral contraceptives or any exogenous sex steroid, or any chronic systemic or ocular medications were excluded. Moreover, patients with a Body Mass Index (BMI) of over 40 kg/m2 were excluded. The first group included women in the reproductive age of 20-39 years (n=40). These women had a regular menstrual cycle. The other groups included older women as follows: the second group (40-49 years), the third group (50-59 years, n=40), and the fourth group (>60 years, n=20). Follicular Stimulating Hormone (FSH) was ordered for women older than 40 years to confirm the menopause status, which is defined as an FSH level greater than 30 IU/ml and the absence of period (amenorrhoea) for at least a year. Assessment of blood pressure at the time of recruitment and BMI was conducted for all participants.
2.2. Assessment of Superficial Capillary Plexus using Optical Coherence Tomography (OCT) Angiography (OCT-A)
All subjects underwent retinal blood flow assessment using Optical Coherence Tomography (OCT) Angiography (Maestro2, Topcon Healthcare, Japan) and the examination was performed by a single skilled optometrist. It is a non-invasive, high-resolution, and in-depth visualization of retinal and eye microstructures without contact or the need for contrast dye injection. IMAGEnet 6 for OCT is the dedicated software for 3D OCT-A that provides static information on blood flow. It is used to save and browse the images and examine data photographed and measured by 3D OCT-A. The OCT-A works by the principle of diffractive particle movement detection of red blood cells on sequential OCT B-scans repeatedly performed at the exact retina location. Automated imaging for the Superficial Retinal Vasculature (SVP) layer was obtained for each patient with comprehensive segmentation to provide the Capillary Density Index (CDI) of the four quadrants, 1.55 mm radium around the fovea. The ratio between the high signal area and the low signal area is displayed in color and numbers. Three readings estimated the proportion of the superficial retinal vascular layers.
2.3. Data Analysis
Data were expressed as the mean and standard deviation for continuous age, BMI, and PB variables. While FSH, a non-normally distributed variable, was depicted as a median and confidence interval. The correlation between age and FSH level was performed using Spearman's rank correlation coefficient. The mean comparison between different regions of the SVP layer was performed using the one-way Analysis of Variance (ANOVA) with Tukey's multiple comparisons post-test. All statistical analyses were conductedusing EXCEL, version 16.74, and GraphPad Prism, version 9.5.1 for Mac (GraphPad Software, San Diego, California USA, www.graphpad.com).
3. RESULTS
3.1. Participants Characteristics
After determining the best-corrected Snellen visual acuity and refraction, an experienced ophthalmologist performed ocular examinations using Slit-lamp and direct ophthalmoscopy to ensure the integrity of the ocular surface and the retina. Subjects were recruited when they had a BCVA of 20/25 or better, were free of ocular disease, and had no history of chronic systemic disease, ocular surgery, or trauma. None of the participants smoked or took medications that could interfere with blood flow readings. However, data show that older women in groups 3 and 4 have significantly higher systolic pressure (p=0.004) compared to young women aged 20-39 years.
Table 1. Shows participants' characteristics, including age, BMI, and BP measurements at the time of recruitment and retinal blood measurement.
| Characteristics |
Group 1 (20- 39 years) |
Group 2 (40-49 years) |
Group 3 (50-59 years) |
Group 4 (≥60 years) |
|---|---|---|---|---|
| Sample size (n) | 40 | 24 | 40 | 20 |
| Age (mean ± SD) | 31.2 ± 4.7 | 45.4 ± 2.9 | 52.1 ± 2.6 | 63.9 ± 2.3 |
| Body Mass Index, kg/m2 (mean ± SD) | 25.1 ± 2.2 | 27.4 ± 2.9 | 27.1 ± 3.6 | 27.1 ± 3.1 |
| Blood pressure (mmHg) | ||||
| Mean systolic ± SD, (range) | 122.8 ± 6.2 (115-135) |
123.2 ± 14 (110-154) |
130 ± 10 (113-150) |
133 ± 11.9 (117-150) |
| Mean diastolic ± SD, mmHg (range) |
80.4 ± 6.5 (70-96) |
80 ± 5.3 (74-94) |
81 ± 8.2 (65-101) |
82 ± 6.3 (70-91) |
3.2. Determination of FSH Levels
Comparison of medians and 95% confidence intervals (95% CI) of serum FSH levels (IU/L) were determined in three age groups above 40 years. In women aged 40-49 years (n=20), the median FSH =12.72 with 95% CI 3.2-17.9 and was found to be significantly lower compared to group 2 (p<0.001) and the third group women (p<0.0001). In women aged 50-59 (n=36), the FSH= 31.71 with 95% CI 21.5-52. For older women of ≥60 years (n=20), the median FSH=86.33 with 95% CI 80-95.87, which was found to be significantly higher than the second group (p<0.0001). The median and 95% CI of FSH levels in the three groups are shown in Fig. (1). As expected, as women get older, their FSH levels increase significantly. The Spearman correlation coefficient (r) shows this relationship, with the r value =0.8 with a 95% CI (0.67-0.86), which was found to be significant (p<0.0001).
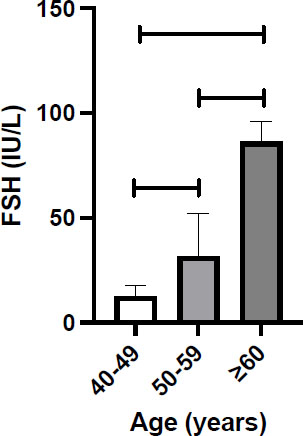
Levels of FSH (IU/L) in women aged ≥40 years.
3.3. Assessment of Superficial Vascular Plexus (SVP)
The average Capillary Density Index (CDI) for all sections of the Superficial Vascular Plexus (SVP) from pooled data was 47.9±5.6%. The average CDI for the four quadrants was 48.4±6.6%, 48.6±5.6%, 48.03±4.9%, and 46.4±4.6% for the superior, inferior, temporal, and nasal, respectively. The CDI was significantly lower (p<0.01) in the nasal quadrant (46.4±4.6%), with a mean difference of 2.01±0.71% and 2.2±0.71% versus the superior and inferior quadrants, respectively. A similar pattern was observed when data were stratified based on age groups with a lower CDI ratio in the nasal quadrant seen in all age groups. However, it was found to be statistically significant (p<0.001) in the age group 40-49 years (Fig. 2). It is worth mentioning that the interindividual variability within this group was higher than in the rest age groups. To investigate the role of blood pressure on the CDI, data was stratified according to the systolic blood pressure as follows: less than 120 mmHg, 120-129 mmHg, 130-139 mmHg, and more than 140 mmHg. As demonstrated in Table 2, there were no statistical differences within and between the quadrants with increased blood pressure levels.
| Systolic Blood Pressure (mmHg) | Capillary Density Index (mean ± SD) | p-value | |||
|---|---|---|---|---|---|
|
Superior
(n=35) |
Inferior
(n=31) |
Temporal
(n=32) |
Nasal
(n=26) |
||
| <120 | 48.4 ± 4.4 | 47.8 ± 4.7 | 48.3 ± 3.9 | 46 ± 4.1 | 0.07 |
| 120-129 | 48.6 ± 6.2 | 49.7 ± 6.7 | 47.2 ± 4.8 | 46.3 ± 4.9 | 0.09 |
| 130-139 | 47.5 ± 9.1 | 48.9 ± 5.8 | 48.4 ± 5.0 | 46.5 ± 4.0 | 0.44 |
| >140 | 49.4 ± 6.4 | 47.8 ± 6.4 | 48.3 ± 6.2 | 46.9 ± 5.8 | 0.56 |
| p -value | 0.77 | 0.50 | 0.7 | 0.8 | - |
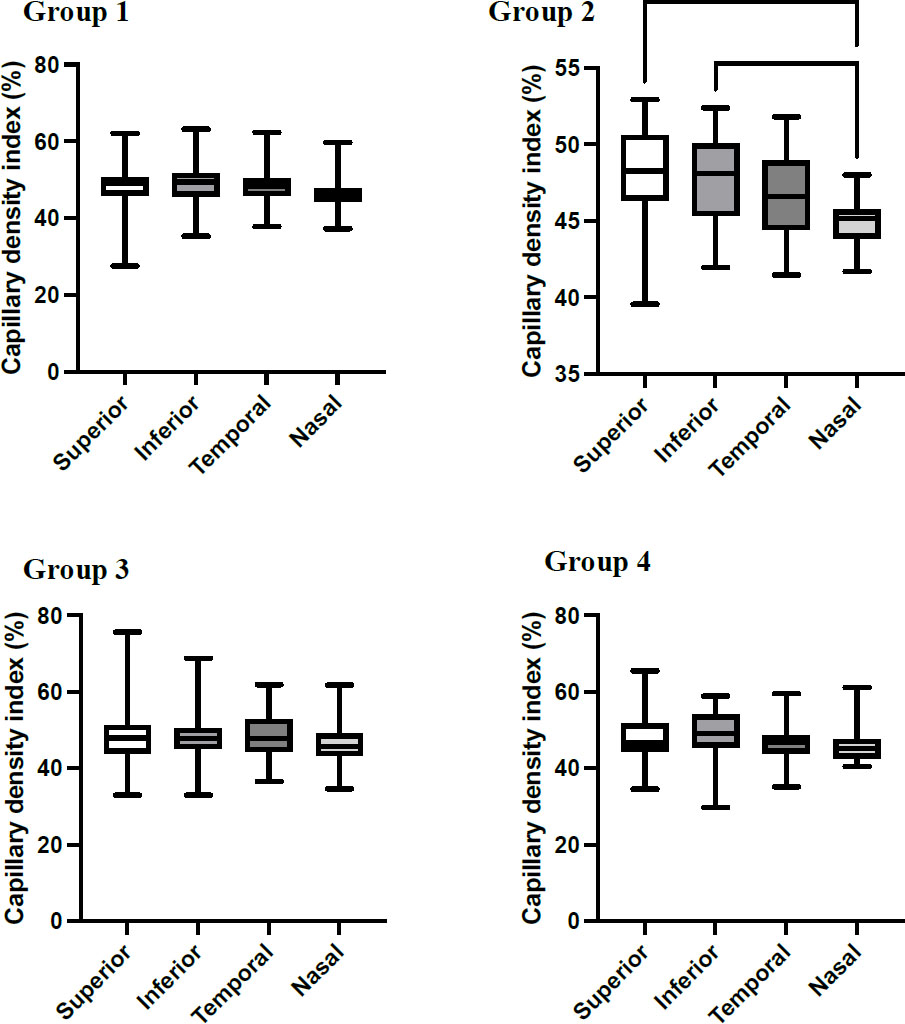
The capillary density index (%) in each quadrant for different age groups. The mean CDI for all quadrants within the age group was performed using one-way ANOVA followed by Tukey’s comparison post-test at p<0.05. Group 1: women aged 20-39 years; Group 2: women aged 40-49; Group 3: women aged 50-59; and Group 4: women aged ≥60.
3.4. Effect of Body Mass Index on the Capillary Density Index
To investigate the influence of BMI on the retinal blood flow, the participants were classified into healthy (n=44), overweight (n=65), and obese (15). Although the nasal quadrant showed lower CDI in all BMI categories, there was no significant difference in the SVP quadrants among each BMI class (Fig. 3).
4. DISCUSSION
This cross-sectional study provides data for retinal blood flow in 124 healthy women of 22-69 years of age using the OCT-angiography. Previous studies reported the predominance effect of estrogen as a vasodilator and neuroprotective mediator in which its deprivation increases the prevalence and onset of ocular disorders in postmenopausal women [7, 11-15]. In agreement with this context, this study examined women of different age groups to detect abnormalities in the retinal blood flow associated with aging and possibly attributed to low hormonal status, considering factors including systemic blood pressure and body weight. The capillary density in the Superficial Vascular Plexus (SVP) was assessed in four quadrants: temporal, inferior, nasal, and superior. The SVP comprises superficial arterioles and venules and provides oxygen and nutrients to the nerve fiber layer, ganglion cell layer, and the inner plexiform layer, and vessel density reduction in this plexus can present in glaucoma [17]. The results showed comparable capillary density in all four quadrants in all age groups, giving no evidence for pathological insults. However, there was a reduction trend in the nasal quadrant that is not statistically significant except in the age group of 40-49 years compared to superior and inferior quadrants. In an attempt to explain this, Hammer et al. attributed the lower oxygen saturation in
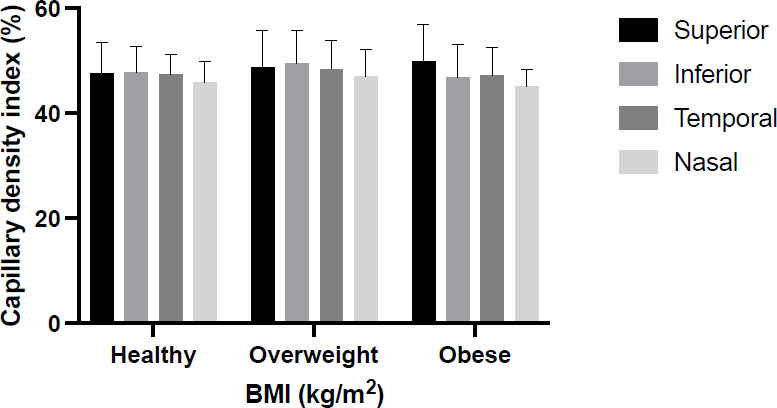
The capillary density index (%) in each quadrant for healthy (BMI=18.5-24.9), overweight (25-29.9), and obese women (BMI= ≥30). The mean CDI for all quadrants within the BMI \ was performed using one-way ANOVA followed by Tukey’s comparison post-test at p<0.05.
the central part of the retina and the macula compared to the nasal quadrant to the higher metabolic needs in these areas [18]. They further explained that from the anatomic point of view, the diameter of branch vessels is higher in the superior and inferior quadrants than in the nasal. Therefore, blood flow is expected to be higher. In agreement with the current result of Isik et al. OCTA data revealed that age does not affect the superficial vessel density in healthy subjects aged 21-60 years [19]. On the contrary, Lavia et al. found that in healthy individuals (aged 22 to 76), retinal vessel density decreases in the SVP with age, regardless of gender [20].However, interestingly and consistent with our results, they reported significantly higher density in the superior and inferior sectors compared with the nasal quadrant.
Previous studies have shown decreased total retinal blood flow with increasing age using different techniques. Early research by Grunwald et al. indicated that retinal macular blood flow and velocity were reduced in healthy subjects aged 20-78 using the blue field simulation technique [21]. Moreover, in a middle-aged group of Chinese subjects, the mean parafoveal flow and mean vessel area density decreased with age [22]. In another study that included healthy eyes of various ethnic backgrounds whose ages ranged from 9 to 88 years old of both genders, vessel density was significantly smaller in the superficial retinal capillary plexus with increasing age [23]. A similar conclusion has been reported by Shahlaee et al. in the foveal and parafoveal regions of healthy eyes, where there was a negative correlation between vascular density and age regardless of sex [24]. Su et al. suggested that the superficial retinal capillary plexus vessel densities began to decrease at approximately 40 years of age.However, vessel densities decreased earlier and more rapidly in the deep plexus at the age of 35 in both genders and then remained stable in the females after the age of 50 years [25].
In disease states like diabetes mellitus, Kushner-Lenhoff et al. reported the capillary density derived by OCT-angiography correlates with the clinical severity of diabetic retinopathy and aids in classifying the retinopathy stage in clinical settings [26]. Moreover, the study revealed that for every 0.001 unit decrease in density, the odds of having any retinopathy compared to control subjects increases by 18%, which makes it a valuable discriminatory parameter [26]. Therefore, the superficial retinal vessel density has been considered a method of detecting and monitoring disease progression when retinal thickness appears normal [27].
It is worth mentioning that gonadal hormones, including androgen, estrogen, and progesterone receptors, are expressed in the ocular structures of men and women. However, age and sex may influence the distribution of these receptors [28-30]. Post-mortem examination of human eyes revealed that expression of estrogen receptor-alpha is present in the retina and retinal pigment epithelium of women of reproductive age but not in that of postmenopausal women or men [31]. The risk of cataracts, AMD, and glaucoma was higher for women once they reached menopause, suggesting the protective effect of estrogen on women's health [32, 33].
Earlier studies have reported that hormonal therapy increases retinal and optic head blood flow in postmenopausal women and reduces the vascular resistance distal to the ophthalmic artery to levels of premenopausal women [8, 34]. The current study confirmed the post-menopausal status of participants of ≥40 years with an FSH level >30 IU/L, together with direct questions to women regarding the menstruation status. The findings did not support the assumption that age associated with menses cessation could lead to abnormalities in the retinal blood flow, as the capillary density for women in this age group was comparable to younger peers. This is in line with the findings of Yaprak et al. who assessed the vessel density in the superficial and deep capillary plexus in pre and postmenopausal women using swept-source OCT-A and reported similar vessel density in both groups [35]. However, the study reported that postmenopausal women had significantly lower choriocapillaris vessel density and a wider foveal avascular zone [35].
To investigate if the women in this study have factors that may affect the ocular blood flow, the CDI was compared across different levels of systemic blood pressure and according to Body Mass Index (BMI).The current study found that higher blood pressure levels did not influence the capillary density across the quadrants. However, other studies have reported that patients with hypertension decreased macular vessel density in the superficial and deep venous plexus [36]. Chua et al. reported that patients with uncontrolled hypertension with poor estimated glomerular filtration rate significantly reduced capillary density in the deep plexus but not the superficial plexus [37].
Our data showed no association between capillary density and BMI in normal, overweight, or obese women. Isik et al. found an inverse correlation between BMI and mean macular thickness [19]. However, the authors indicated that the BMI values were considered normal, and the population sample included only young individuals with a mean age of 39.5 ±11.2. On the contrary, a recent article by Ding et al. demonstrated a positive association between generalized obesity and vascular density. The study included normal, overweight, and obese Chinese subjects with no history of ocular disease. Using OCT-A, the macular vessel density was calculated in the superficial and deep capillary plexus, and it was concluded that the vascular density increases as the BMI increases [38].
Our data is limited by the fact that there is a variation in the sample size under each BMI class to allow a statistical comparison and correlation. In addition, assessment of other parameters, including deep retinal layer and retinal blood vessel diameter, could provide a comprehensive evaluation of sex hormones' effect on the retinal blood flow. Moreover, measurement of blood levels of sex hormones, including luteinizing hormone, estradiol, and progesterone for pre and postmenopausal women, could offer more association with ophthalmic findings.
CONCLUSION
Evidence from this study indicates that healthy postmenopausal women have a normal capillary density index in the superficial plexus quadrants, comparable to younger women, and are unaffected by systemic hypertension and high body mass index. Further study is needed to compare the superficial and deep retinal capillary plexus in health and disease states in a larger population.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: A.A., M.A.: Study conception and design; H.A.: Data collection. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BMI | = Body Mass Index |
| BP | = Blood Pressure |
| CDI | = Capillary Density Index |
| FSH | = Follicular Stimulating Hormone |
| OCTA | = Optical Coherence Tomography Angiography |
| AMD | = Age-related Macular Diseases |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Institutional Review Board of King Saud University, Saudi Arabia (protocol code E-23-7606; approval date 22 March 2023).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


