All published articles of this journal are available on ScienceDirect.
Efficacy of Transconjunctival Botulinum Toxin Type A Injection for the Treatment of Thyroid Eye Disease-Induced Upper Eyelid Retraction
Abstract
Introduction
This study aimed to investigate the efficacy and side effects of transconjunctival botulinum toxin type A (BTX-A) injection for treating upper eyelid retraction (ULR) associated with thyroid eye disease (TED).
Methods
This clinical before–after case series involved 30 eyes of 26 patients with TED-induced ULR in the congestive and fibrotic stages. BTX-A (Dysport®) was injected into the levator palpebrae superioris through the conjunctiva at 10, 20, and 30 UI, depending on the baseline margin reflex distance 1 (MRD1) level. Patients were monitored and followed up at 1 week, 1, 3, and 6 months postinjections. Complications and patients’ feelings after treatment were documented.
Results
The mean MRD1 decreased to 3.5 ± 2.08 mm after a week. The success rate (MRD1 within a normal range) was highest 3 months postinjection at 66.67%. Ptosis was the only complication with a 60% incidence rate and lasted not >3 months. In the significant majority of patients, the symptoms reduced, and they were satisfied with their appearance posttreatment (73.07%).
Discussion
This method is applied to manage ULR due to TED, especially in patients who are unwilling for surgeries with a high success rate, reducing discomforts and improving patient satisfaction with their appearance.
Conclusion
Transconjunctival BTX-A injection is an effective and safe method for treating TED-induced ULR. However, ptosis is a prevalent transient complication.
1. INTRODUCTION
Thyroid eye disease (TED), also known as Graves’ ophthalmopathy, is an autoimmune condition caused by antibodies directed against receptors that are present in the thyroid cells and on the surface of the orbital cells. The incidence of TED is rare, with 16 cases per 100,000 females, compared to 2.9 cases per 100,000 males. It is clinically characterized by soft tissue swelling, proptosis, eyelid retraction, restrictive myopathy, corneal ulcers, and compressive optic neuropathy. In particular, upper eyelid retraction (ULR) is the most prevalent symptom, accounting for approximately 90%, appearing in a congestive (active) phase and lasting through the fibrotic (stable) disease stage. It significantly affects the quality of life because it not only brings an unacceptable appearance but also causes exposure to keratopathy with tearing, photophobia, grittiness, and even corneal ulcers, resulting in vision loss. Conventional treatment involves conservation and surgery. However, surgical procedures cannot be performed in the active stage as well as in the presence of significant risks with unpredictable outcomes. Artificial tears can be used during the active phase to relieve discomfort from 6 to 12 months. However, it is not sufficient for some patients. This poses a challenge for ophthalmologists to discover new treatments that lower the upper eyelid promptly. Nowadays, botulinum toxin type A (BTX-A), which paralyzes muscle fibers by preventing acetylcholine release at the motor end plates, is injected into the upper eyelid levator and Muller’s muscles to weaken them, resulting in eyelid drooping. Various studies have been conducted on BTX-A in treating this condition with high efficacy and fewer complications [1-3]. Furthermore, in Vietnam, no previous study has focused on this treatment. Thus, the present study aimed to investigate the efficacy, safety, and complications of BTX-A in treating TED-induced ULR through the transconjunctival approach.
2. MATERIALS AND METHODS
Due to the rare incidence of TED, this research is a case series study, including all patients diagnosed with TED-induced ULR who came to the Eye Hospital of HCM City from January 2020 to December 2020.
2.1. Inclusion Criteria
The inclusion criteria are as follows:
- Unilateral or bilateral TED-induced ULR in the congestive or fibrotic stage.
- Older than 18 years.
2.2. Exclusion Criteria
The exclusion criteria are as follows:
• Optic neuropathy.
• Severe corneal ulcers.
• Requires urgent orbital decompression.
• Other ocular conditions or surgeries.
• Other systemic diseases.
• Pregnancy or breastfeeding.
• Quit follow-up visit. Study Design: It is a before-after case series. Research Process: Patients were consulted about the treatment benefits and complications. All study participants signed the informed consent form and received the treatment. BTX-A (Dysport®) was injected into the levator palpebrae superioris through the conjunctiva (Fig. 1) with a dose based on the baseline margin reflex distance 1 (MRD1) level (Table 1). Patients were re-examined at 1 week, 1, 3, and 6 months to assess the visual acuity, proptosis, extraocular movements, MRD1, fissure height (FH), complications, and symptoms experienced, such as pain, burning, tearing, grittiness, photophobia, and eye appearance. Data were processed and presented by Stata 13.0 and Excel 2013.
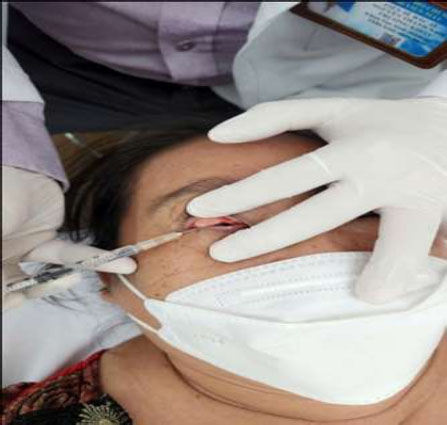
Transconjunctival injection of BTX-A was administered into the Muller’s muscle at the central superior tarsal border.
| MRD1 | BTX-A (UI) |
|---|---|
| > 5 - < 7 mm | 10 |
| 7 – 8 mm | 20 |
| > 8 mm | 30 |
3. RESULTS
3.1. Epidemiology
This study included 30 eyes of 26 patients due to the rare incidence of TED (8 males and 18 females). Such a number of cases is consistent with the rarity of the disease, as mentioned previously. The mean age of the patients was 38.12 ± 9.85 years (range: 22–56 years). The majority age of patients was 30–40 years (46.15%), followed by 40–50 years (23.07%), and the lowest was 20–30 years and 50–60 years (15.38%). The mean duration of ULR was 10.46 ± 13.34 months (range: 2–72 months), with the 6–12 months group having the highest rate (65.39%), followed by <6 months (26.92%), and the lowest was >12 months (7.69%). No linear correlation was observed between the age and the duration.
3.2. Treatment
The visual acuity in both the injected eyes and contralateral eyes did not change over time. The mean visual acuity was 0.85 ± 0.11 in the injected eyes (lowest: 5/10, highest: 10/10) and 0.9 ± 0.13 in the contralateral eyes (lowest: 5/10, highest: 10/10), with no difference between them. The protrusion of the globe did not change. It was 17.33 ± 2.77 mm (range: 12–25 mm) in retracted eyelids and 16.23 ± 2.05 mm (range: 12–21 mm) in the normal eyelids. In the study, only two patients had extraocular movement disorders before the injection (Table 2), and these conditions did not change during treatment.
3.3. MRD1
In 26 patients, 30 eyelids required injection. The moderate degree (MRD1 of ≥ 7 to ≤ 8 mm) accounted for 56.67%, followed by mild (MRD1 of > 5 to < 7 mm) and severe (MRD1 of > 8 mm) degrees at 33.33% and 10%, respectively. The baseline MRD1 of the treated eyes was not correlated with the duration of retraction or the age of the patient. Post-injection, MRD1 reached the lowest level at 1 week, then gradually increased at 1, 3, and 6 months (Table 3).
MRD1 at each examination was statistically different (p < 0.05). After one week of follow-up, 60% of the eyes showed ptosis, 26.67% appeared normal, and 13.33% remained retracted and required an additional injection. After 1 month of follow-up, among 25 eyes, 24% showed ptosis, 64% appeared normal, and 12% remained retracted. After 3 months, 22 eyes were followed up, 90.91% and 9.09% of which were normal and remained retracted, respectively. After 6 months, 20 eyes were followed up, 80% and 20% of which were normal and remained retracted, respectively (Fig. 2).
Before treatment, a clear difference was observed between the MRD1 in untreated eyes and treated eyes at 2.91 ± 0.97 mm (range: 1–5 mm) (for each patient). Post-injection, MRD1 in normal eyes exhibited a slight increase, mostly at 1 week. The mean difference between the normal and retraced eyelids was decreased, especially at 1 month (Table 3).
3.4. Success Rate
The success rate was 26.67% after a week of treatment. This rate continued to increase after 1 month (53.33%) and reached a peak of 66.67% at 3 months. However, it decreased to 53.33% after 6 months. The logistic regression model analysis with odds ratio revealed no baseline epidemiological or clinical characteristics related to the outcome after treatment.
| - | - | MR | LR | SR | IR | SO | IO |
|---|---|---|---|---|---|---|---|
| Patient 1 | OD | -1 | 0 | -1 | -1 | 0 | 0 |
| OS | -1 | -1 | -3 | -2 | 0 | 0 | |
| Patient 2 | OD | -1 | -2 | -3 | -1 | -1 | -3 |
| OS | -1 | -2 | -4 | -1 | -1 | -4 |
| - | Pretreatment | 1 Week | 1 Month | 3 Months | 6 Months |
|---|---|---|---|---|---|
| Injected eyes | 6.93 ± 0.91 | 3.43 ± 2.14 | 4.2 ± 1.66 | 4.95 ± 1.13 | 5.1 ± 0.55 |
| Contralateral eyes | 4.09 ± 0.68 | 4.45 ± 0.86 | 4.38 ± 0.59 | 4.28 ± 0.57 | 4.25 ± 0.58 |
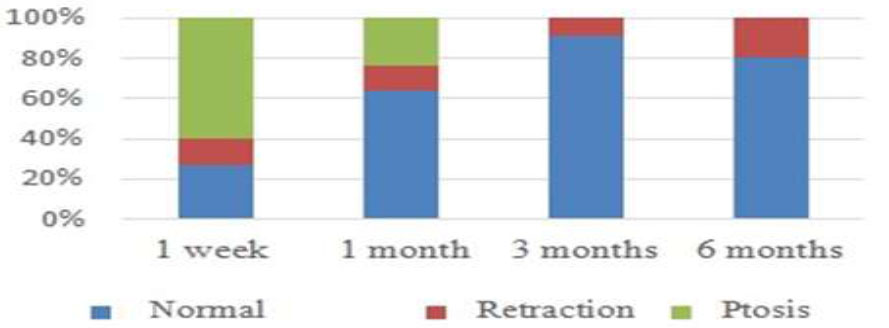
Status of the upper eyelid after drug injection over time.
3.5. FH
Before treatment, FH in the treated eyes was higher than that in the contralateral eyes, ranging from 1 to 4 mm. Post-injection, FH in the affected eyes significantly decreased at 1 week and then slightly increased until 6 months later. However, the FH at 6 months remained much lower than the baseline (in cases where reinjection was not required). FH has no significant change for the contralateral eyes. FH moderately increased at 1 week before decreasing. The FH difference between normal and treated eyes decreased after treatment, significantly 1-month post-injection, similar to the MRD1 level (Table 4).
3.6. Complications
The ptosis rate was 60% after a week. Most cases were mild or moderate (38.89% for each level), and the severe degree was 22.22%. The ptosis rate dramatically dropped to 24% in 1 month, consisting of mild, moderate, and severe degrees at 50%, 16.67%, and 33.33%, respectively. No droopy eyelids were reported from the third month onward. Overall, ptosis occurred in the first week post-injection with three different degrees, then gradually decreased and lasted up to 3 months (Fig. 3).
Besides ptosis, the study reported no other side effects, such as double vision, eyelid edema, or infection. In terms of patient feelings, uncomfortable symptoms included pain, burning sensation, tearing, grittiness, and photophobia. No symptoms got worse after treatment, and most of them improved or were stable (Fig. 4). Regarding the eye appearance, 73.07% of patients were satisfied with the results and accepted re-injections when the eyelid retraction occurred again. The remaining were dissatisfied either because the eyelids did not achieve the desired treatment outcome or the duration of normal eyelid positioning was too short.
| - | Pretreatment | 1 Week | 1 Month | 3 Months | 6 Months |
|---|---|---|---|---|---|
| Injected eyes | 11.73 ± 1.26 | 8.5 ± 2.47 | 9.12 ± 2.05 | 9.77 ± 0.81 | 10 ± 0.73 |
| Contralateral eyes | 9.05 ± 1.21 | 9.36 ± 1.36 | 9.19 ± 1.17 | 8.89 ± 0.96 | 9 ± 0.89 |
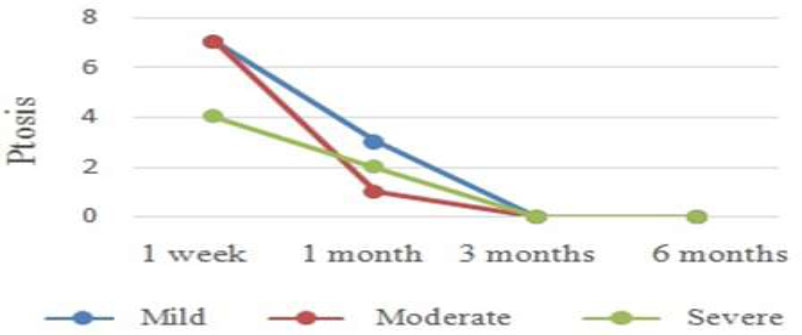
Progression of ptosis.
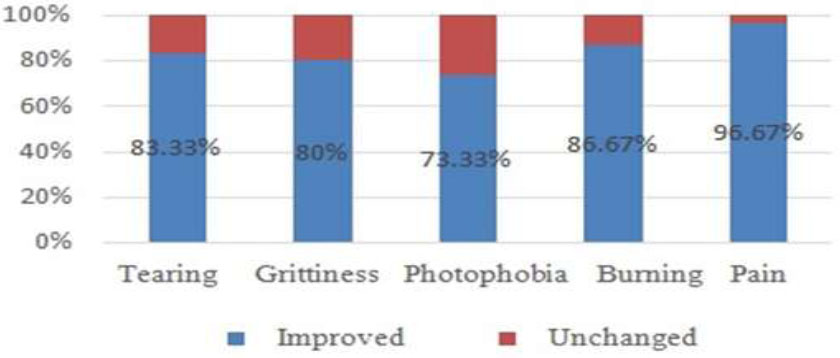
Evaluation of symptoms 6 months after treatment.
4. DISCUSSION
4.1. Epidemiology
According to the literature, TED is more prevalent in females than in males. Our study included more female patients than male patients, similar to the reports of other authors [4-7].
Regarding the age of patients, we selected over 18 years so that patients could be independent in deciding treatment methods and study participation. As reported, ULR of thyroid disease occurs at any age, most predominantly between 30 and 50 years. Therefore, the mean age in our study was similar to that in the literature and studies by Shih et al. [8], Costa et al. [3], and Salour et al. [9]. Furthermore, Ozturk reported that BTX-A could be performed in adolescence (15–17 years of age) [10].
Most authors performed injections when TED was stable for at least 6 months. However, Morgenstern and Shen applied this method in an active phase and revealed its effectiveness [11, 12]. Costa and Young then compared the results of BTX-A in both stages and demonstrated that the effect in the stable phase lasted longer than in the active one [3, 13]. All of these reports were a foundation for us to apply this method promptly when patients were diagnosed with ULR due to TED. Most of our patients were in the fibrotic stage, had a long-term retraction, and required an intervention. In particular, a patient with a retracted eyelid for 6 years responded very well to treatment after only 1 injection (>6 months follow-up).
4.2. Treatment
We found no difference in the visual acuity between the injected eyes and the contralateral eyes, and no no change was observed before and after treatment. Meanwhile, only Sharon reported quite high visual acuity in the treated eyes, as also reported in our study [14]. This proved that BTX-A did not influence the visual acuity.
We revealed no change in the protrusion before and after treatment. The difference between the treated eyes and contralateral eyes was not significant, which was not reported previously. Therefore, the treatment may not affect the protrusion and vice versa.
Two patients had extraocular movement disorders before treatment. Patient no. 1 had a short-term retraction (4 months), with mild restriction of the superior and inferior rectus muscles of the right eye, mild-moderate restriction of the four rectus muscles of the left eye, and unaffected two obliques. This was consistent with the “I'M SLOW” rule, wherein the inferior rectus muscle is affected first, followed by the medial, superior, levator, lateral rectus, and oblique. Patient no. 2 experienced disorders in all extraocular muscles of both eyes, ranging from mild to severe. This is related to a long-term retraction (14 months) without any treatment. However, all of the disorders remained unchanged postinjection, indicating that BTX-A had no or extremely minimal effect on these muscles. Other authors revealed that patients had normal eye movements before treatment, so they could easily estimate the complications. They reported a weakness of the superior rectus muscles with low rates, causing transient diplopia (under 1 month), attributed to BTX-A acting on Muller’s muscle and affecting the superior rectus muscle. Sharon revealed no significant difference between the transcutaneous and transconjunctival injections, causing this muscle weakness [14].
The MRD1 level and the proportion of pretreatment degree of eyelid retraction in our study were similar to those reported by other authors because most of the patients were in the fibrotic stage. After treatment, the MRD1 level in our study was similar to the others, although the method and the injection time (the stage of TED) were different. In the first 1–2 weeks, MRD1 markedly decreased at approximately 3.5 mm, then rapidly increased to approximately 4.5 mm at 1 month. It proved once again that BTX-A was effective in reducing MRD1 of eyelid retraction, and the effect was maintained for at least 6 months thereafter.
Three patients had five upper eyelids that remained retracted after 1 week of injection. MRD1 increased in a patient with unilateral lid retraction because she was in a congestive stage, and a retraction appeared in the contralateral eye and lower eyelids of both eyes. This patient received two additional BTX-A injections but was not successful, probably because the patient refused hyperthyroidism treatment. Patient no. 2, who received bilateral injections, demonstrated unchanged MRD1 in the right eye and a decreased MRD1 from moderate to mild in the left eye. This was explained by Hering’s law, wherein the innervation for the levator palpebrae superioris muscles is equal in both eyes, and if the upper eyelid is dropped with BTX-A, the nerve distribution for this eyelid will increase against the ptosis; hence, the contralateral upper lid is simultaneously raised. Therefore, the MRD1 of the right eye remained unchanged instead of decreasing when the left eyelid dropped (even though the target was not as desired) in this patient. After additional injections in both eyes, the target was achieved, and the effect was maintained for 4 months. Similarly, patient no. 3 had bilateral retractions, with severe effects in one eye (causing ptosis), resulting in excessive retraction in the other eye, which increased MRD1. This patient received an additional injection in the retracted eyelid, and the outcome was good, which lasted for 3 months.
All the other re-retraction cases were determined in patients who received unilateral injections but still showed some improvements. All of them agreed to receive additional injections and achieved a normal upper eyelid, lasting approximately 4 months. The unsuccessful outcomes were because the method that we applied was new, the injection technique of the first few cases was not accurate in getting the BTX-A into the correct target tissue, and the injection dose was not suitable for each patient.
The mean pre-injection MRD1 for contralateral eyes in our study was slightly higher than that in a study by Costa [3], whereas other authors reported no such data. This difference could be explained by two reasons. First, we selected to inject with MRD1 of > 5 mm (because the normal MRD1 is 4–5 mm), whereas in the study by Costa, MRD1 of 5 mm was selected. Second, TED-related myasthenia gravis may have occurred. Therefore, the MRD1 level in the study by Costa was < 3 mm in some cases. After 1–2 weeks of treatment, MRD1 increased more in both studies and reached quite similar levels, especially in the stable-stage group of a study by Costa. In the active-stage group, it increased, but it was not significant. This was explained by Hering’s law. MRD1 in the treated eyes increased when the BTX-A concentration decreased, thereby reducing MRD1 levels in the contralateral eyes. However, they remained slightly higher than the baseline (within normal range), alongside improved MRD1 in the treated eyes after 6 months.
4.3. Success Rate
The success rate in our study was highest at 3 months (66.67%) and remained effective up to 6 months (53.33%) after the first injection. However, patients with the same MRD1 level demonstrated different outcomes. We conducted a logistic regression analysis but determined no initial factors affecting the results. Studies by other authors did not mention this issue. Therefore, future studies with larger sample sizes and control groups are warranted to focus on this research question.
4.4. Fissure Height
Only one study mentioned FH, reporting a higher level in injected eyes compared with our study, both before and after treatment. However, the variation in FH and FH according to MRD1 was comparable between the two studies. At 1–2 weeks postinjection, FH significantly decreased then slightly increased, but remained lower than the baseline up to 6 months thereafter. This indicated that the efficacy of BTX-A was similar in different samples and different stages of TED and did not affect the lower eyelid (MRD2 remained unchanged). FH in the contralateral eyes was not significantly lower in our study as compared to the study by Costa. When analyzing the sample of that study, we found higher MRD2 levels in normal eyes than in retracted eyes, indicating that MRD1 and MRD2 may not be homologous in TED.
4.5. Complications
Other studies revealed that ptosis was an unavoidable side effect despite different doses and injection methods, ranging from 3.7% to 40% [2, 14]. However, ptosis was only transient and lasted up to 1 month. Kohn and Sharon indicated that the transconjunctival injection had a higher rate of ptosis than the transcutaneous injection [4, 14]. Our rate of ptosis was much higher than reported by others because we selected the transconjunctival injection with a high dose of BTX-A for a higher success rate and longer-lasting effect. Therefore, to obtain the highest effectiveness and lowest side effects, the drug concentration needs to be adjusted to be more suitable, and this will be the goal for future studies.
In addition to ptosis, other side effects, such as diplopia and ecchymosis, were also reported in various studies. In particular, the rate of bruising was found to be rare, only reported in one study with an incidence rate of 4.2% [3] when injected through the skin. The rate of double vision ranged from 3.7% to 11.1%, transient under a month, mostly in studies that used transcutaneous injections [8, 14]. In contrast, we did not record any diplopia or ecchymosis cases, indicating an accurate technique.
4.6. Patients’ Feelings
Only studies by Traisk (2001), Sharon (2019), Sandra (2023), and our group assessed the patient’s feelings. Traisk indicated that sensations, such as pain, burning, tearing, grittiness, and photophobia, improved after treatment (>60%), and patients were satisfied with the cosmetic results (88.89%) [5, 14, 7]. Diltenman and Kozaki revealed that the transcutaneous injection was more painful than the transconjunctival injection [6, 15]. We found that uncomfortable symptoms decreased after the treatment (>70%), with no pain, and the majority of patients were satisfied. Consequently, besides the eyelid-lowering effect, BTX injection also helped patients significantly reduce the discomfort caused by dry eyes.
CONCLUSION
Transconjunctival BTX-A injection into the upper eyelid is not only a quick and safe procedure but also economical and easily accessible. This method is applied to manage TED-induced ULR in both the congestive and fibrotic stages, especially in patients who are unwilling to undergo surgery. Additionally, this treatment can be repeated after 3 months while surgery is still delayed. It exhibits a high success rate, reduces discomfort, and improves patient satisfaction with their appearance. Besides the eyelid-lowering effect, BTX-A injection also helps patients significantly reduce the discomfort caused by dry eyes. Last but not least, ptosis is considered a transient complication, demonstrating no serious consequences.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: T.N.N.: Study concept or design; T.V.: Data analysis or interpretation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| BTX-A | = Botulinum toxin type A |
| FH | = Fissure height |
| ULR | = Upper eyelid retraction |
| TED | = Thyroid eye disease |
| MRD1 | = Margin reflex distance 1 |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ho Chi Minh City Eye Hospital Ethical committee approved this clinical study (No. 199, March 17th, 2020).
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this published article.
ACKNOWLEDGEMENTS
Declared none.


