RESEARCH ARTICLE
Present and New Treatment Strategies in the Management of Glaucoma
Kolko M*, 1, 2, 3
Article Information
Identifiers and Pagination:
Year: 2015Volume: 9
Issue: Suppl 1: M5
First Page: 89
Last Page: 100
Publisher ID: TOOPHTJ-9-89
DOI: 10.2174/1874364101509010089
Article History:
Received Date: 28/3/2015Revision Received Date: 30/3/2015
Acceptance Date: 30/3/2015
Electronic publication date: 15 /5/2015
Collection year: 2015

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Glaucoma is a neurodegenerative disease characterized by retinal ganglion cell (RGC) death and axonal loss. It remains a major cause of blindness worldwide. All current modalities of treatment are focused on lowering intraocular pressure (IOP), and it is evident that increased IOP is an important risk factor for progression of the disease. However, it is clear that a significant number of glaucoma patients show disease progression despite of pressure lowering treatments. Much attention has been given to the development of neuroprotective treatment strategies, but the identification of such has been hampered by lack of understanding of the etiology of glaucoma. Hence, in spite of many attempts no neuroprotective drug has yet been clinically approved. Even though neuroprotection is without doubt an important treatment strategy, many glaucoma subjects are diagnosed after substantial loss of RGCs. In this matter, recent approaches aim to rescue RGCs and regenerate axons in order to restore visual function in glaucoma. The present review seeks to provide an overview of the present and new treatment strategies in the management of glaucoma. The treatment strategies are divided into current available glaucoma medications, new pressure lowering targets, prospective neuroprotective interventions, and finally possible neuroregenrative strategies.
INTRODUCTION
Glaucoma refers to a group of eye conditions, which cause progressive damage to the optic nerve, retinal ganglion cell (RGC) death, and characteristic damage to the visual field. According to The World Health Organization, glaucoma accounted for 2 percent of visual impairment and 8 percent of global blindness in 2010, and the number of glaucoma patients is estimated to increase due to a growing population [1]. The classification of glaucoma relies on the appearance and obstruction of the drainage pathway. In open angle glaucoma (OAG) the drainage pathway appears normal and in angle-closure glaucoma (ACG) the drainage pathway is obstructed. Glaucoma is also classified according to whether it is primary or associated with detectable comorbidity, secondary glaucoma. The most common subtype of glaucoma is primary OAG (POAG). Despite the normal clinical appearance of the drainage pathway the aqueous outflow is restricted in most POAG and referred to as high-tension glaucoma (HTG). Hence, glaucoma is associated with an increase in intraocular pressure (IOP), and to date IOP lowering drugs remain the only clinically validated treatment of glaucoma [2]. Despite the significant importance of IOP in the risk of glaucoma progression, it is recognized that elevated IOP appears in the absence of the characteristic optic nerve changes (ocular hypertension (OHT)) and conversely glaucomatous optic nerve damage appears in the absence of an elevated IOP (low-tension glaucoma (LTG)).
Therefore, despite the fact that IOP lowering interventions reduce the risk of progression and delay the disease onset of glaucoma, the pathogenesis is controversial and not completely understood. In this matter non-IOP-dependent risk factors appear to be responsible for approximately 30-70 percent of glaucoma cases [3-6].
The present review seeks to 1) briefly summarize the current treatment strategies for glaucoma, 2) discuss future treatment strategies for glaucoma i.e. new targets for IOP-lowering, targets for neuroprotection, and targets for neuroregeneration.
CURRENT TREATMENT STRATEGIES FOR GLAUCOMA
Although glaucoma is a complex and poorly understood disorder, the primary goal of therapy is lowering IOP [2]. Hence, lowering IOP by 20 - 40 percent has been shown to reduce the rate of progressive visual field loss by half [7,8].
The first anti-glaucomatous drop was introduced in 1875 and there are currently several types of IOP-lowering eye drops used to treat glaucoma (Table 1). In addition, two systemic IOP-lowering drugs are available (Table 2). The eye drops include β-blockers, carbonic anhydrase inhibitors, prostaglandin analogs, α2-adrenergic agonists, and parasympathomimetic drugs. In addition to the pure form, the eye-drops often come as combined drops. To date fixed-combination eye-drops include prostaglandin analogs/β-blockers, carbonic anhydrase inhibitors/β-blockers, and α2-adrenergic agonists/β-blockers. Finally, a combination of carbonic anhydrase inhibitors /α2-adrenergic agonists was approved by the United States Food and Drug Administration in April 2013, and as an exception a triple fixed combination of prostaglandin analogs/α2-adrenergic agonists/β-blockers is available in Mexico.
Time line for the introduction of glaucoma medication.
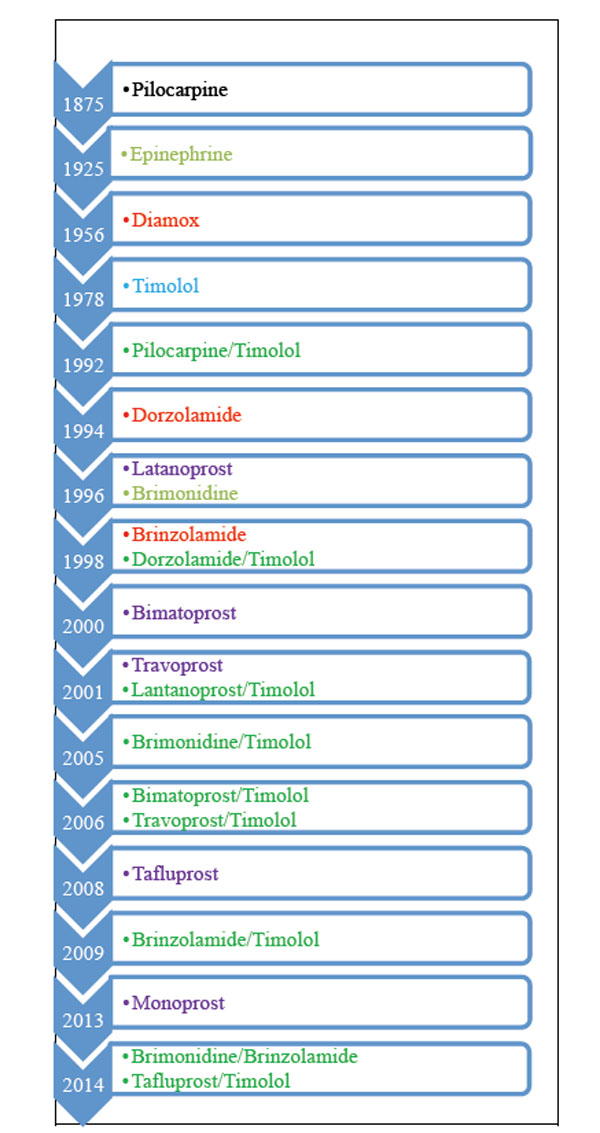 |
IOP Lowering medication and year of introduction. Modified from European Glaucoma Society Therminology and Guidelines for Glaucoma, 4th edition.
Current treatment strategies for glaucoma.
ß-blockers
|
The list of glaucomatous medication is based on the current available products in Denmark.
* Requires special permission or is not available in Denmark.
Targets for new treatment strategies in the management of glaucoma.
| IOP Lowering Strategies | Neuroprotective Strategies | Neuroregenerative Strategies |
|---|---|---|
|
Increasing Trabecular Meshwork Outflow ROCK Endothelin-1 Nitric Oxide TGF-ß CTGF Adenosine Angiopoietin-like7 molecules Cannabinoids Cochlin Latrunculins Melatonin Ghrelin Increasing the Uveoscleral Outflow Angiotensin II Serotonin Ghrelin Cannabinoids Decreasing Aqueous Humor Production Forskolin Serotonin Cannabinoids Angiotensin II |
Exitotoxicity NMDA antagonists (Memantine) Modulation of Müller cells Oxidative stress Antioxidants (α-tocopherol) Ginkgo Biloba Mitochondrial Dysfunction Mitochondrial targeted antioxidants (Q10) Inflammation- Abnormal Immune Response TNF-α Biological response modifiers (Ethanrecept) Agmatine Modulation of T-cell reaction (Cop-1) Modulation of PLA2-induced inflammation Protein Misfolding Agents targeting Aß Heat shock proteins Glial Cell Modulation TGF-ß, CNTF, PDGF Other Pathways Estradiol Statins Erythropoietin |
Cell Repair Inflammatory stimulation (CNTF) Gene Therapy (Nogo Receptor interference) Surgical Approaches Lens Injury Stem Cell Therapy CNTF-secreting RPE cells MSC transplantation |
Current treatment strategies. Abbreviations are: Rho-associated Kinase (ROCK), Tumor Growth Factor-ß (TGF-ß), Connective Tissue Growth Factor (CTGF), Tumor Necrosis Factor-α (TNF-α), Phospholipase A2 (PLA2), Amyloid-ß (Aß), Ciliary Neurotrophic Factor (CNTF), Platelet-derived Growth Factor (PDGF), Retinal Pigment Epithelial Cells (RPE), Mesenchymal Stem Cells (MSC).
Over all, the current available glaucoma eye-drops all seek to decrease the IOP. They can be grouped into therapeutic agents that decrease the production of aqueous humor production and/or increase the drainage through the trabecular meshwork (TM) and/or increase uveoscleral outflow.
ß-Blockers
ß-blockers reduce the production of aqueous humor. In addition, some ß-blockers contain α1 blocking effects (levobunolol and nipradilol) [9-11], which reduce IOP by an acceleration of the uveoscleral outflow. Ocular adverse reactions include conjunctival allergies, conjunctival injection and corneal epithelium disorder. Additionally, corneal sensitivity may be reduced in case of the selective ß1-blocker, betaxolol, due to its membrane-stabilizing effect. In contrast, another ß-blocker, carteolol, has intrinsic sympathomimetic activity and therefore no reduced corneal sensitivity [12]. One major challenge for the use of ß-blockers is their frequent systemic adverse effect due to their activation of both ß1- and ß2-receptors. In this matter adverse effects of the respiratory system by ß2-blockers include worsening of asthma attacks and chronic obstructive pulmonary disease. To prevent the ß2-related side effects betaxolol can be used in cases with respiratory issues [13]. The most critical adverse effects of ß1-blockage are reduced heart rate and reduced cardiac contractility. Hence, ß-blockers should be used with caution in patients with slow or irregular heartbeat or congestive heart failure. Finally, adverse effects from the use of ß-blockers include depression, impotence, and drowsiness.
Carbonic Anhydrase Inhibitors
Carbonic anhydrase inhibitor (CAI)s reduce IOP by inhibiting the ciliary epithelium and controlling aqueous formation. Systemic CAIs have been used since 1956, but are associated with a high incidence of adverse reactions, including dysesthesia of the fingers and around the lips, frequent urination, lassitude, anorexia, weight reduction, kidney stones, metabolic acidosis, and hematopoietic cell restraint anemia [14]. Since 1994 a topical CAI has been available (Table 1). Even though the adverse effects are much less compared to systemic administered CAIs, topical CAIs have some ocular adverse reactions such as conjunctival allergy and hyperemia [14]. Due to the fairly acidic pH, CAIs generally cause ocular irritation. Moreover, carbon anhydrase naturally exists in the endothelial cells, and CAIs should be used with caution in patients with corneal endothelial disorders [15]. No significant systemic adverse reactions have been associated with the use of CAIs.
Prostaglandin Analogs
Prostaglandins lower IOP by accelerating the uveoscleral outflow. The most common adverse effects are eye redness or irritation, a change in eye color (mostly in hazel or green eyes) [16,17], and an increase in thickness and number of eyelashes [18]. In addition, prostaglandin administration has been reported to recur corneal epithelium herpes, and should therefore be used with caution in these patients [19]. No significant systemic adverse reactions have been associated with the use of prostaglandins.
Sympathomimetic Drugs
Sympathomimetic drugs act on α2-receptors and activate G protein-coupled receptors, thereby reducing cAMP. In this way the production of aqueous humor production is reduced and the uveoscleral outflow increased. Allergic reactions frequently occur with this class of medication. Side effects may further include irregular heart rate, elevated blood pressure, headaches, blurred vision, fatigue, dry mouth, and redness in or around the eye [20]. A randomized trial of the α2-receptor agonist, brimonidine, versus the ß-blocker, timolol, found evidence of a less likely visual field progression in patients treated with brimonidine compared to timolol, thereby indicating a neuroprotective role of α2 agonists [21].
Parasympathomimetic Drugs (Miotics)
Parasympathomimetic drugs are cholinergic agents that cause the pupil to become much smaller in diameter and help increase the rate of fluid drainage from the eye. The most common ocular side effects include headache, red eyes, miosis-caused visual field constriction, and night vision loss. Systemic miotics may cause excessive salivation and tearing, sweating, diarrhea, vomiting, and slowed heartbeat [22].
In addition to the main therapeutic agents all eye drops contain additives, which stabilize the solution/suspension, and/or extend the life of the drugs. The draw back of additives is the ocular adverse reactions that follow these. Hence, more anti-glaucomatous drops now come in preservative free versions.
Another issue concerning adherence and adverse reactions is the increasing introduction of generic glaucoma medication. Within defined tolerances, generic drugs are “equivalent” to original, patented medications. There is, however, room for variability and error in manufacture, packaging, and the adjuvants can vary considerably. Because generic formulations do not necessarily undergo clinical testing, physicians and patients need to make sure that the medications are equally effective in real-life use. Even though the challenge of adverse reactions from preservatives, and the challenge of an increasing number of generic products are important issues, these topics are beyond the scope of this review.
FUTURE TREATMENT STRATEGIES FOR GLAUCOMA
Many mechanisms have been proposed to address the pathogenesis of glaucoma. However, none seems to characterize the disease sufficiently, and the multifactorial etiologies of glaucoma become a fundamental challenge in the development of new treatment strategies. Nevertheless elevated IOP together with yet-to-be elucidated cellular and molecular changes result in glaucomatous neurodegeneration. In this aspect treatment strategies can be grouped into 1) IOP lowering strategies, 2) neuroprotective strategies, and 3) neuroregenerative strategies (Table 3).
NEW TREATMENT STRATEGIES FOR IOP LOWERING
It is clear that multiple factors give rise to glaucomatous damage, and it is recognized that the most evident risk factor is IOP. The cause of elevated IOP in POAG is thought to be due to an increased accumulation of extracellular matrix material (ECM) in the TM. From the current approved glaucoma medications only prostaglandin analogues may have a role on modulation of the molecular changes that occur in the TM of glaucoma patients. Hence, prostaglandins may induce stimulation of matrix metalloproteinases, and in this way lead to increased spacing between the ciliary muscle bundles [23,24].
Since the importance of IOP in the progression of glaucoma is evident, current new strategies target IOP lowering pathways. Among these exist two main approaches that try to increase the outflow facilities in the TM. The first strategy seeks to modulate the contractility of TM. It has been shown that TM possesses smooth muscle cell-like properties, and that TMs contractile properties can be regulated by several enzymes [25-29]. The second therapeutic concept includes alteration in the behavior of TM, and strategies to affect the shape and loosen the cell-to-cell junction and/or cell-to-ECM adhesion within the TM have become experimental targets for lowering IOP [30]. In addition, new therapeutic targets aim to decrease aqueous humor production or to improve the uveoscleral outflow by different subcellular pathways from those already existing [31-33].
TREATMENT STRATEGIES TO INCREASE TRABECULAR MESHWORK OUTFLOW
Rho-Associated Kinase
The Rho signaling pathway, mediated through Rho-associated kinase (ROCK), plays a major role in regulation of smooth muscle contraction. Therefore, ROCK inhibitors may enhance aqueous drainage by acting on the actin cytoskeleton and cellular motility in the TM, Schlemms canal and ciliary muscle [34,35]. Multiple animal studies have shown an IOP reduction by topical use of ROCK inhibitors, and more ROCK inhibitors have been and are currently tested in clinical trials for glaucoma and ocular hypertension. The current (or completed without posted results) clinical trials include K 115 (phase III, #JapicCTI-111565), AMA0076 (phase II, #NCT01693315), AR-13324 (PG324) (phase III, #NCT02057575), AR12286 (Phase II, #NCT02174991/ #NCT02173223) [36].
Although much attention is given to ROCK inhibition no drugs are yet on the market, and during the clinical trials concerns have been raised. Hence, ROCK inhibition in humans seems to elicit different IOP responses compared to animals and have resulted in side effects including moderate to severe hyperemia, vascular disorders and other system problems [37-39]. Overall, ROCK inhibitors, however, offer a potentially exciting alternative to the prostaglandin analogues, and if one tested target overcomes the side effects other beneficial effects of ROCK inhibition have been shown. Hence, in addition to ROCK inhibitors modulative effect on the TM, ROCK inhibition furthermore offers neuroprotective effects as well as enhances blood flow to the optic nerve [40].
Endothelin-1
Another modulator of TM is endothelin-1 (ET-1), and a significant correlation has been found between IOP and ET-1[41-43]. Hence, increased ET-1 levels may stimulate contraction of both TM cells and the ECM, and thereby reduce TM outflow [42,44]. In addition to reduced TM outflow increased levels of ET-1 have been implicated in vascular dysregulation. In this matter raised concentrations of ET-1 in glaucoma patients may lead to vasoconstriction by stimulation of ET-1 receptors on the vascular smooth muscle cells [45,46]. In addition to a decreased IOP, ET-1 receptor inhibition has been shown to have neuroprotective effects [47]. Overall, an ET-1 receptor antagonist constitutes a potential treatment target to manage IOP reduction, which may also favor the vascular regulation and RGC survival.
Nitric Oxide
Nitric oxide (NO) has been implicated in more mechanisms related to glaucoma such as auto regulation [48], RGC survival and death [49], and low-grade inflammation [50]. Hence, dependent on the mechanism, it may be beneficial or damaging to glaucomatous progression. In relation to the IOP, NO agonists induce relaxation of TM cells and thereby increase the outflow [51,52].
Transforming Growth Factor-ß
Elevated levels of transforming growth factor-ß (TGF-β) have been identified in the anterior chamber of glaucomatous eyes [53], and increased levels of TGF-β have been postulated to increase the risk of glaucoma [54]. TGF-β has also been shown to directly cause increased IOP [55]. It is believed that this occurs through complex interactions with the TM, leading to decreased aqueous humor outflow [55,56].
Beside a consistent beneficial effect by TGF-β inhibition on IOP, the role of TGF-β in RGC maintenance has contrasting beneficial effects. Hence, TGF-β has multiple functions and among others, significant evidence shows a neuroprotective effect of TGF-β [57-59]. Therefore, future potential clinical relevance of TGF-β inhibition to reduce IOP requires attention to potential side effects.
Connective Tissue Growth Factor
Recent studies have described a complex relationship between increased connective tissue growth factor (CTGF) expression, TGF-β activity, and fibrotic pathogenesis [60-62], thereby highlighting the complex signaling interplay between CTGF and TGF-β that results in increased fibrosis in the TM. Hence, interfering with CTGF expression may therefore prove beneficial in the treatment of glaucoma.
Adenosine
Adenosine and several adenosine derivatives increase and/or decrease IOP via modulation of G protein coupled receptors [63]. There are four adenosine receptor subtypes known as A1, A2a, A2b, and A3. Activation of A1, A2a and A3 agonists and A3 antagonists has been shown to lower IOP by remodeling the ECM, through activation of metalloproteinases, and thereby increase TM outflow [32]. A3 receptor antagonists have been shown to prevent adenosine-induced activation of chloride channels of the ciliary non-pigmented epithelial cells followed by an IOP reduction [37,64]. Clinical trials are ongoing, but so far no significant results have been reported (Phase II, #NCT01917383) [37].
Other New Targets to Increase Trabecular Meshwork Outflow
In the search for targets to increase TM outflow, multiple molecules are in consideration. Among these are angiopoietin-like7 (ANGPTL7), a member of the ANGPTL family, which has been shown to increase TM outflow [65]. Other potential molecules that could be targeted in the regulation of TM outflow are cannabinoids, cochlin, latrunculins, melatonin, and ghrelin [66].
TREATMENT STRATEGIES TO INCREASE THE UVEOSCLERAL OUTFLOW
Angiotensin II
Compounds that increase angiotensin-converting enzyme 2- (ACE2) activity and further the formation of angiotensin (1-7) are new options as anti-glaucomatous drugs in addition to classical ACE inhibitors and AT1 receptor blockers. Furthermore, drugs that activate the angiotensin receptor types, Mas receptors, directly have also been suggested as possible targets for IOP lowering [67].
TREATMENT STRATEGIES TO DECREASE AQUEOUS HUMOR PRODUCTION
Forskolin
Foskolin is a lipid-soluble compound that activates cAMP in the ciliary epithelium thereby reducing the aqueous humor production. It has been shown to be able to decrease IOP after topical application by a mechanism that is not used by the other drugs [71].
Other New Targets to Decrease Aqueous Humor Production
In addition to foskolin, targets such as serotonin, cannabinoids and angiotensin II have also been suggested as potential drugs that decrease the aqueous humor production [66].
NON-IOP DEPENDENT TREATMENT STRATEGIES
Beyond IOP, it is recognized that many other factors are associated with an increased risk of developing glaucoma. Hence, a significant number of glaucoma patients continue to lose vision despite successful IOP control, and it has been estimated that IOP-independent risk factors appear to be responsible for approximately 30-70 percent of glaucoma cases [4,6,72,73].
A variety of non-IOP dependent risk factors are being proposed and in order to categorize and summarize the current knowledge, this review seeks to group these into two major groups i.e. neuroprotective- and neuroregenerative- treatment strategies.
NEUROPROTECTIVE TREATMENT STRATEGIES
In order to simplify the complexity of the proposed new neuroprotective treatment strategies, these can be grouped into targets that interfere with excitotoxicity, oxidative stress, mitochondrial dysfunction, inflammation - abnormal immune response, protein misfolding, and glial cell modulation. Obviously, such a division of treatment strategies is not definite and most targets will interfere with more pathways.
Excitotoxicity
Exitotoxicity refers to the pathological process by which neurons are damaged by the overactivation of glutamate receptors. In glaucoma, the initial insult to RGCs has been suggested to lead to elevated levels of extracellular glutamate [74-76]. In line with this, a chronic elevation of glutamate concentrations in the inner eye has been shown in glaucoma patients [77-79]. As a consequence of increased levels of glutamate, ionotropic N-methyl-D-aspartate (NMDA) receptors are overstimulated, resulting in a massive influx of calcium into the neurons, thereby causing glutamate-mediated RGC death. Strategies to modify glutamate-induced neurotoxicity have been widely studied, and in particular the NMDA antagonist, memantine, has been shown to be a highly effective neuroprotective agent in both acute and chronic animal models of RGC death [80-82].
In the well known memantine clinical trial no significant benefit was found in the memantine-treated group compared to the patients receiving placebo [83]. However, in line with the substantive evidence that glutamate promotes RGC death via NMDA over activation, the design and the clinical end points of the memantine study have been questioned and it still remains uncertain whether glutamate excitotoxicity is an efficient target for therapeutical intervention in glaucoma [83].
In addition to increased secretion of glutamate, reduced clearance by the Müller cells may account for excitotoxicity [84,85]. Hence, instead of, or together with, targeting the ionotropic glutamate receptors, the homeostasis of Müller cells could be a valuable target in the understanding of the homeostasis in the inner retina. Furthermore, the neurovascular junction (the interaction between pericytes, Müller cells and inner retinal neurons) may account for attention, since a dysfunctional nutrient and oxygen supply may affect the Müller cells ability to remove excess glutamate from the intercellular space and thereby their ability to protect the RGCs [86,87].
Oxidative Stress
Oxidative stress reflects an imbalance between the production of reactive oxygen species (ROS) and the cells ability to readily detoxify the reactive intermediates or to repair the resulting damage. Significant evidence has shown that oxidative stress plays a role in RGC death in glaucoma, and the concept of antioxidants as a neuroprotective treatment strategy is widely accepted [88-92]. Among the most studied antioxidants, vitamin E (α-tocopherol) and gingko biloba have been shown to ameliorate NMDA-induced RGC death. Vitamin E acts as a scavenger of peroxyl radicals. Although some studies have suggested a decreased rate of glaucomatous progression in patients receiving vitamin E the long-term results are still lacking [93,94]. To further elucidate a role of vitamin E in the treatment of glaucoma, current studies are suggesting that vitamin E, release from contact lenses may be used in preventing ROS-induced glaucomatous damage [95,96].
Gingko biloba is an extract from Ginkgo biloba leaves. It increases blood flow and has been shown to have a free radical scavenger property [97]. In addition, gingko biloba has been shown to interfere with glutamate signaling and to preserve mitochondrial metabolism [98-100]. The precise mechanism by which gingko biloba interferes with RGC homeostasis is still not fully understood, however, the studied literature on gingko biloba is in favor of a possible beneficial effect on RGC survival [101].
Mitochondrial Dysfunction
In line with the role of oxidative stress in the pathogenesis of glaucoma, increasing evidence points to a mitochondrial dysfunction in glaucoma [102-105]. Even though many antioxidants have shown promising effects on RGC survival, many of them lack specificity to mitochondria, the key regulator of ROS production. Hence, compounds that specifically target the mitochondria have been suggested to be more beneficial. Among mitochondrial-targeted antioxidants, coenzyme Q10 (Q10) is one of the most studied targets, and in animal models, Q10 protects RGCs after ischemia [106,107] and oxidative stress [108]. To date, no human clinical trials have been published on the use of Q10 in glaucoma.
Inflammation - Abnormal Immune Response
It is widely accepted that glaucomatous neurodegeneration comes with an activation of glial cells and accompanying production of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α). TNF-α is secreted by damaged glial cells and through the binding of TNF-receptor-1 (TNF-R1) it causes apoptotic RGC death [109]. However, the binding of TNF-R1 also triggers the activation of transcription factor NF-KB, a cell survival pathway [110,111]. Overall, growing evidence exists on the role of TNF-α in RGC death [112-115], but opposing consequences of TNF-α -signaling challenge any strategy for neuroprotection [50,116].
More biologic response modifiers, such as ethanrecept, have been shown to possess promising results as neuroprotective agents by inhibiting TNF-α-induced RGC damage [117].
Another anti-inflammatory agent, agmatine, has been shown to protect RGCs from TNF-α-induced cell death [118,119]. In addition, it is known that agmatine protects neurons from apoptosis after exposure to NMDA and glutamate [120]. Finally, agmatine has a role as an α2-adrenergic agonist, and thus, can suppress RGC death by neuroprotective mechanisms, and also protect RGCs by lowering the IOP [121].
The inflammatory process in glaucoma has been found to be associated with pro-inflammatory activities mediated in part by T cell activity. In this aspect, Cop-1, a synthetic peptide polymer, has been shown to modulate this T-cell reaction by attenuating the normal inflammatory response. Furthermore, it has been shown experimentally that glutamate injections into the eye result in T cell reaction [122,123], and in this aspect, Cop-1 immunization has shown some protection against RGC death [122-124].
A recognized inflammatory pathway is initiated by phospholipase A2 (PLA2) activity. Hence, PLA2 activation leads to the cyclooxygenase-2 (COX-2)-mediated prostaglandin synthesis. Studies have shown an induction of COX-2 in RGCs in response to elevated IOP [125,126]. In line with this, COX-2 inhibition has been found to rescue RGCs [127].
The precursor of COX-2 medicated prostaglandin synthesis is arachidonic acid (AA), which is released by PLA2-cleavage of phospholipids. Both AA and PLA2 have been shown to be involved in RGC maintenance. In this matter AA was found to ameliorate RGC death [128]. Opposing results have revealed a negative role of PLA2 on neuronal survival. Hence, PLA2 has been shown to act synergistically with glutamate-induced exitotoxicity [129]. Moreover, levels of the PLA2 subgroup sPLA2-IIA was found to be increased in the aqueous humor of glaucoma patients [130,131], and studies from the brain have revealed that increased levels of PLA2 instigate neuronal cell death [132-134].
Protein Misfolding
Protein aggregation is a prominent factor in neurodegenative disorders such as Alzheimer disease (AD). In AD, the peptide amyloid-ß (Aß) has been implicated in the neuropathology, and growing evidence suggests that Aß aggregation is also involved in the development of RGC apoptosis. In line with this experimental studies for glaucoma support the involvement of Aß, and different agents targeting Aß formation have been shown to reduce RGC apoptosis in models of glaucoma [135-138].
In addition to Aß another mechanism of protein aggregation involves heat shock proteins (HSP). HSPs are thought to prevent the aggregation of denatured proteins and immunohistochemical analysis has demonstrated that HSP-60 and -27 are greater in glaucomatous eyes compared to non glaucomatous eyes of humans [139], thereby suggesting that HSPs may be part of a defense mechanism that is activated in glaucomatous optic neuropathy. Furthermore, HSP72 upregulation has been shown to correlate with increased survival of RGCs in a rat model of acute glaucoma [140]. In glaucoma the HSP-inducer, geranylgeraylacetone, has furthermore been shown to induce HSP72 and thereby ameliorate RGC death. Even though no current HSP-inducing drugs have been administered to glaucoma patients, it may be a future target for preventing glaucomatous damage [141,142].
Glial Cell Modulation
Much attention has been given to RGC maintenance in the search for new treatment targets in glaucoma. However, it is clear, that the surrounding cells tightly regulate RGC homeostasis. Hence, in the non-myelinated region of the RGCs, Müller cells and astrocytes (macroglial cells) are the major glial cells to provide support, as well as to create the interface between RGCs and blood vessels. They remove excess glutamate from the synapse thereby preventing exitotoxicity [87,143], and help to maintain ion homeostasis and extracellular pH. In addition, macroglial cells liberate cytokines such as TGF [144], ciliary neurotrophic factor (CNTF) [145], and platelet-derived growth factor [146]. Hence, modulation of macroglial cell activity may therefore be a key target in the understanding of RGC protection [147,148].
Other Pathways
Considerable evidence exists on estrogen as a neuroprotective drug, and a recent study has provided strong evidence that topical estrogen drops are neuroprotective in a rodent model of glaucoma [149]. Another suggested drug to prevent glaucomatous damage is statins. Hence, long-term use of statins has been shown to be associated with a reduced risk of glaucoma [150,151]. Finally, the glycoprotein, erythropoietin (EPO), has been suggested to be a potential therapeutic neuroprotectant in glaucoma [152,153].
NEUROREGENERATIVE TREATMENT STRATEGIES
Growing evidence suggests cell repair or cell-replacement therapy as a new treatment approach. Within the potential group of cell repair treatment strategies, axonal growth has become a target for investigation. Furthermore, surgical approaches to enhance regenerative capacity of RGCs have been suggested. Finally, stem cells hold great promise for neurodegenerative disorders such as glaucoma.
Cell Repair
Although the critical first step in the treatment of glaucoma is enhancing RGC survival, a significant number of patients will be diagnosed at a later stage by which their axons have already been injured. In these cases, preventing apoptosis will not be sufficient and the ideal therapies should encourage axon regeneration to rebuild connections from the RGCs to the brain.
In this aspect, more molecules have been shown to possess regenerative properties due to their inflammatory stimulation [154-156]. Among these, CNTF has been shown to induce axonal growth and thereby suggested to provide a new neuroregenative approach [147,157,158].
Because of the promising experimental results, a phase-I trial in glaucoma has recently been performed (Phase I, # NCT01408472). Through search, no final outcome is yet available, however, although the study is a phase 1 trial and designed to evaluate safety in the patient population being studied, any hint of efficacy would be expected to drive investigation in later-phase trials.
In addition to CNTF, studies have shown a potential role of Nogo-66 receptor (NgR)1 therapy for glaucoma to prevent RGC death and promote optic nerve axonal regeneration. Hence, inhibition of NgR1 by RNA interference or by transfection of the dominant-negative form of NgR1, has been shown to stimulate optic nerve axon regrowth. Furthermore, knockout of NgR1 has been shown effective for enhancing axonal regeneration after optic nerve crush [159,160].
Surgical Approaches for Neuroregeneration
Penetrating injury as well as lens injury has been suggested to result in the release of low-grade inflammatory molecules, which secondly leads to axonal regeneration [161,162]. In order to consider this strategy in humans, a number of issues would have to be considered including a rapid formation of cataract, infection, etc. Hence, pursuing the molecular basis of the effect may prove more realistic for translation to human glaucoma treatment.
Stem Cell Therapy
Stem cell therapy holds great promise for neurodegenerative diseases and emerging studies try to identify the use of stem cells in experimental glaucoma. Substantial evidence has correlated neurotrophic factor deprivation with RGC death and new therapies aim to supplement these [163]. To avoid repeated injections of growth factors, cell-based delivery of neurotrophic factors have been proposed. In this matter, an ongoing phase-I clinical trial for glaucoma is using genetically modified CNTF-secreting retinal pigment epithelium cells (Phase I, #NCT01408472). In addition, transplantation with mesenchymal stem cells (MSC) has been suggested, since these produce neurotrophic factors [164-167]. Furthermore, intracranial human umbilical cord blood MSC transplantation has been shown to protect RGC and to induce axonal regeneration [168]. Overall, the neuroprotective and neuroregenerative effect of MSCs on RGC survival is evident, and currently, a clinical trial using bone marrow-derived MSCs on glaucoma is processed. The outcome of this study is expected in 2017 (Phase I, #NCT01920867).
CONCLUSION
The present review highlights current treatment strategies and possible future ways to rescue and regenerate RGCs. Glaucoma remains a major cause of blindness worldwide. Various new targets to treat glaucoma have been suggested, but to date the only available glaucoma medication is IOP lowering compounds, which are only decreasing the rate of progression. Hence, no cure for glaucoma exists. The identification of new therapeutic targets has been hampered by lack of understanding of the etiology of glaucoma, and the limited number of animal models available that likely represent only a small subset of human glaucoma cases. Since glaucoma may be a spectrum of different pathologies leading to the same endpoint, the outcome from clinical trials may be lost in the diversification of etiologies. In order to identify future RGC rescuing drugs, attention must be given to the study design. Hence, a more stringent patient selection and more efficient outcome measurements will be necessary to document the effectiveness of these.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The time spent for writing the present review was granted by The Danish Council for Independent Research|Medical Sciences and the Velux Foundation for whom I am grateful. I thank Kurt Bang for providing information of currents treatment strategies, and for his enthusiasm of the present manuscript. My apologies to all colleagues whose work I have not included.






