All published articles of this journal are available on ScienceDirect.
The Spectrum of Microbial Keratitis: An Updated Review
Abstract
Background:
In microbial keratitis, infection of the cornea can threaten vision through permanent corneal scarring and even perforation resulting in the loss of the eye. A literature review was conducted by Karsten, Watson and Foster (2012) to determine the spectrum of microbial keratitis. Since this publication, there have been over 2600 articles published investigating the causative pathogens of microbial keratitis.
Objective:
To determine the current spectrum of possible pathogens implicated in microbial keratitis relative to the 2012 study.
Methods:
An exhaustive literature review was conducted of all the peer-reviewed articles reporting on microbial pathogens implicated in keratitis. Databases including MEDLINE, EMBASE, Scopus and Web of Science were searched utilising their entire year limits (1950-2019).
Results:
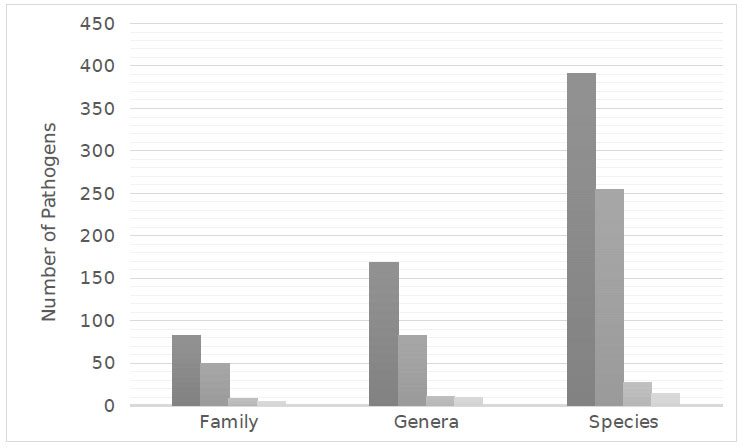
Six-hundred and eighty-eight species representing 271 genera from 145 families were implicated in microbial keratitis. Fungal pathogens, though less frequent than bacteria, demonstrated the greatest diversity with 393 species from 169 genera that were found to cause microbial keratitis. There were 254 species of bacteria from 82 genera, 27 species of amoeba from 11 genera, and 14 species of virus from 9 genera, which were also identified as pathogens of microbial keratitis.
Conclusion:
The spectrum of pathogens implicated in microbial keratitis is extremely diverse. Bacteria were most commonly encountered and in comparison, to the review published in 2012, further 456 pathogens have been identified as causative pathogens of microbial keratitis. Therefore, the current review provides an important update on the potential spectrum of microbes, to assist clinicians in the diagnosis and treatment of microbial keratitis.
1. INTRODUCTION
Microbial keratitis is a common infectious disease of the cornea that, if untreated, can have severe consequences [1-3]. Consequently, keratitis is considered an ophthalmic emergency requiring immediate and appropriate anti-microbial treatment to prevent permanent vision loss [4]. Current practice is to combat infection through the application of empiric broad-spectrum anti-microbial therapy, which is instituted immediately after corneal scrapings to combat the infection while cultures are processed [3, 5, 6]. Complications, however, may arise due to misidentification of the microbial cause and subsequent inadequate and/or inappropriate treatment [7, 8]. With definitive identification and treatment, the significant morbidity associated with severe microbial keratitis may be avoided.
In 2012, Karsten, Watson & Foster conducted a literature review investigating the spectrum of pathogens implicated in microbial keratitis. According to their review of the literature, 232 species from 142 genera representing 80 families were implicated in microbial keratitis, with bacterial keratitis, the most common. Since this publication, however, there have been over 2700 articles published investigating the identity and aetiology of causative pathogens of microbial keratitis. Therefore, an updated review is required to provide clinicians with current data on causative pathogens and management of microbial keratitis.
The aim of this study was to determine the current spectrum of possible pathogens implicated in microbial keratitis relative to the 2012 study, as well as the aetiological factors and treatment of this eye-threatening disease.
2. MATERIALS AND METHODS
A review of peer-reviewed articles, case reports and conference submissions reporting microbial keratitis pathogens was conducted. The search strategy; ‘keratitis’, ‘microbial keratitis’, ‘bacterial keratitis’, ‘viral keratitis’, ‘fungal keratitis’, ‘amoebic keratitis’ OR ‘parasitic keratitis’ was used in MEDLINE, EMBASE, Scopus and Web of Science databases within the year limits of each of the databases. Searches were restricted to the English language. The most recent search was conducted in August 2019.
The search strategy generated over 12600 articles, case reports and conference submissions to be reviewed. Publications were reviewed and information reporting the identification of organisms that were implicated in microbial keratitis were abstracted on the basis of (a) organism identity, (b) aetiology and (c) treatment. Pathogens were included in the review only if the method of the article outlined that the pathogen was retrieved from a corneal biopsy or scraping and cultured appropriately. The review objective was restricted to species diversity and did not aim to compile any incidence data.
3. RESULTS AND DISCUSSION
In microbial keratitis, a range of microorganisms, including fungi, bacteria, protozoa, and viruses, have been identified as infectious agents. A comprehensive review of the current literature identified 688 species representing 271 genera from 145 families reported to cause microbial keratitis. According to the literature, fungal keratitis, although less common than bacterial keratitis, demonstrated the greatest diversity of pathogens with 393 species from 169 genera being implicated in causing keratitis. Moreover, 254 species of bacteria from 82 genera, 27 species of amoeba from 11 genera, and 14 species of virus from 9 genera were found to be pathogens of microbial keratitis (Fig 1).
In total, 49 bacterial families were identified; 22 were Gram-positive and 27 Gram-negative with Proteobacteria phylum being the most common (Table 1). Eighty-two fungal families were identified, the most common were filamentous fungi (76 families, Table 2). In viral keratitis, 5 families were reported with the majority of the species belonging to the Herpesviridae family. In amoebic keratitis, 8 families were identified to cause microbial keratitis, the most common was Acanthamoeba species.
The presentation of keratitis is often similar between the pathogens, with photophobia, pain, lacrimation and foreign body sensation all common presenting symptoms [9]. The clinical appearance of the cornea, however, may differ depending on the cause of the keratitis [7, 10].
| Phylum | Number of Identified Families | |
|---|---|---|
| Gram Positive | Actinobacteria | 10 |
| Firmicutes | 12 | |
| Gram Negative | Bacteriodetes | 5 |
| Fusobacteria | 1 | |
| Proteobacteria | 20 | |
| Spirochaetae | 1 |
| Phylum | Number of Identified Families | |
|---|---|---|
| Mycota | Ascomycota | 59 |
| Basiiomycota | 16 | |
| Zygomycota | 5 | |
| Slime Mold | Myoxmycota | 1 |
| Straminipila | Oomycota | 2 |
3.1. Bacterial Keratitis
Bacterial pathogens described in the literature were responsible for a greater proportion of keratitis than mycotic pathogens throughout various populations [11-13]. Bacterial keratitis often occurs in patients with inherent ocular risk factors, such as ocular trauma, contact lens use, or corneal disease [4]. Moreover, with the subsequent increased use of prescription and aesthetic contact lenses, there has been an increased prevalence of bacterial keratitis [14].
In this review of the literature, the Gram-positive bacteria identified were almost equally spread between the phylum Actinobacteria and Firmicutes. However, consistent with Karsten, Watson & Foster (2012), the Gram-negative bacteria identified were largely from the phylum Proteobacteria (Table 1). The most diverse species of bacteria were Nocardia, Staphylococcus, and Streptococcus with 24, 19 and 14 species, respectively (Appendix 1). Gram-positive bacteria such as coagulase-negative Staphylococcal (CoNS) species were more common [4] than Gram-negative bacteria as a cause of bacterial keratitis [3, 15]. Although, in contact lens wearers Pseudomonas aeruginosa was the most common cause [4, 14] (Table 3).
| Family | References | Family | References |
|---|---|---|---|
| Acetobacteraceae | [25] | Listeriaceae | [26] |
| Actinomycetaceae | [27, 28] | Microbacteriaceae | [29, 30] |
| Aerococcaceae | [31-33] | Micrococcaceae | [10, 30, 34-40] |
| Aeromonadaceae | [15, 41, 42] | Moraxellaceae | [30, 43-50] |
| Alcaligenaceae | [12, 33, 42, 51, 52] | Mycobacteriaceae | [47, 53-59] |
| Bacillaceae | [60-64] | Neisseriaceae | [13, 51, 65-67] |
| Bacteroidaceae | [68] | Nocardiaceae | [44, 69-86] |
| Bartonellaceae | [87] | Pasteurellaceae | [41, 44, 46, 88-91] |
| Brevibacteriaceae | [12] | Peptococcaceae | [92] |
| Brucellaceae | [49, 93, 94] | Porphyromonadaceae | [95] |
| Burkholderiaceae | [47, 96, 97] | Propionibacteriaceae | [1, 68, 98, 99] |
| Cardiobacteriaceae | [34] | Prevotellaceae | [68, 100] |
| Carnobacteriaceae | [101] | Pseudomonadaceae | [30, 33, 38, 52, 102-107] |
| Caulobacteraceae | [108] | Rhizobiaceae | [109] |
| Clostridiaceae | [60, 68, 110-112] | Rhodobacteraceae | [113] |
| Comamonadaceae | [55, 98] | Rickettsiaceae | [114] |
| Corynebacteriaceae | [15, 44, 62, 105, 115-121] | Sphingobacteriaceae | [30] |
| Enterobacteriaceae | [33, 34, 43, 52, 61, 65, 102, 104, 105, 117, 122-136] | Spirochaetaceae | [136, 137] |
| Enterococcaceae | [33, 39, 138] | Staphylococcaceae | [10, 15, 23, 33, 34, 36, 39, 43, 46, 52, 60, 138-142] |
| Eubacteriaceae | [143] | Streptococcaceae | [33, 41, 46, 47, 49, 60, 64, 65, 117, 126, 144-146] |
| Flavobacteriaceae | [34, 52, 147-155] | Streptomycetaceae | [131, 156] |
| Fusobacteriaceae | [157] | Tsukamurellaceae | [158, 159] |
| Intrasporangiaceae | [160] | Vibrionaceae | [43, 161] |
| Lactobacillaceae | [162] | Xanthomonadaceae | [46, 98, 163] |
| Leuconostocaceae | [10] | Yersiniaceae | [43, 164] |
| Family | References | Family | References |
|---|---|---|---|
| Ajellomycetaceae | [171, 180, 181] | Hypocreales Incertae sedis | [102, 146, 175] |
| Amphisphaeriaceae | [182] | Hyponectriaceae | [12] |
| Arthrodermataceae | [171, 181, 183-188] | Glomerellaceae | [41, 78, 169, 189-195] |
| Ascodesmiaceae | [186, 196] | Gymnoascaceae | [197] |
| Basidiobolaceae | [187] | Lagenidiaceae | [198] |
| Bionectriaceae | [186] | Lasiosphaeriaceae | [169, 173, 186, 193, 199, 200] |
| Botryosphaeriaceae | [11, 169, 175, 176, 188, 191, 196, 201-209] | Lichtheimiaceae | [193, 204] |
| Cephalothecaceae | [202, 210] | Lophiostomataceae | [211] |
| Ceratobsidiaceae | [169, 175] | Malasseziaceae | [175, 212, 213] |
| Chaetomiaceae | [12, 47, 168, 169, 191, 204, 205, 214] | Massarinaceae | [10, 90] |
| Chaetosphaerellaceae | [175] | Metacapnodiaceae | [186] |
| Chaetosphaeriaceae | [186, 206] | Microascaceae | [28, 29, 47, 174, 175, 186, 189, 191, 196, 212, 215-221] |
| Clavicipitaceae | [222-224] | Montagnulaceae | [225] |
| Coniochaetaceae | [28, 175, 210] | Mucoraceae | [10, 28, 29, 173, 175, 186, 191, 193, 219, 226-228] |
| Cordycipitaceae | [47, 175, 191, 229, 230] | Mycosphaerellaceae | [186, 227, 231, 232] |
| Corticiaceae | [28, 233] | Nectriaceae | [8, 11, 28, 112, 127, 146, 172, 173, 175, 186, 191, 193, 199, 204, 234-250] |
| Corynesporascaceae | [251] | Niessliaceae | [186] |
| Cryptococcaceae | [212, 222, 252, 253] | Onygenaceae | [29, 47, 171, 175] |
| Cunninghamellaceae | [186, 254] | Ophiocordycipitaceae | [255] |
| Cystofilobasidiaceae | [222] | Ophiostomataceae | [228, 256] |
| Davidiellaceae | [115, 204, 247, 257] | Orbiliaceae | [258] |
| Debaryomycetaceae | [259] | Phaeosphaeriaceae | [260] |
| Dermateaceae | [261] | Phycomycetaceae | [28] |
| Diaporthaceae | [262, 263] | Plectosphaerellaceae | [175, 264, 265] |
| Didymellaceae | [12, 92, 175, 191, 266] | Pleosporaceae | [34, 111, 115, 169, 171, 175, 190, 191, 193, 199, 204, 207, 212, 220, 240, 249, 267-280] |
| Dipodascaceae | [175, 210, 281, 282] | Pleurotheciaceae | [79] |
| Dothioraceae | [12, 189, 207, 283] | Polyporaceae | [80] |
| Eremomycetaceae | [175, 284] | Pythiaceae | [47, 81] |
| Gjaerumiaceae | [285] | Saccharomycetaceae | [9, 18, 48, 53, 61, 82-86, 105, 125, 163-170, 175, 191, 212, 222, 236, 259, 286] |
| Helotailes incertae sedis | [187, 287] | Schizophyllaceae | [288] |
| Herpotrichiellaceae | [47, 115, 146, 154, 173, 175, 191, 196, 206, 240, 283, 289-295] | Schizoporaceae | [201] |
| Hypocreaceae | [28, 127, 146, 166, 175, 186, 191, 193, 222, 240, 247, 270, 283, 296] | Sclerotiniaceae | [28] |
Bacterial keratitis often presents within an epithelial defect with surrounding corneal infiltrate and stromal oedema [7]. Inflammation within the anterior chamber associated with the keratitis may include a cellular reaction, flare and/or a hypopyon. Progression of bacterial keratitis and outcome tends to relate to the severity of the presentation as well as the potential risk factors for severe disease such as systemic disease and previous ocular history [4, 16]
The recommendations for empiric therapy for the treatment of bacterial keratitis by the Australian Therapeutic Guidelines are ciprofloxacin 0.3% or ofloxacin 0.3% or fortified cefalotin 5% plus gentamicin 0.9% [17]. Indeed, a Cochrane review found equal efficacy of such available topical antibiotics for bacterial keratitis. However, an increase in the relative risk of minor adverse events, including discomfort or chemical conjunctivitis was found with aminoglycosides and cephalosporins compared with fluoroquinolones, although there was no difference in serious complications [18-22]. Yet, the development of bacterial resistance to antibiotics may lead to the ineffective treatment of bacterial keratitis [23, 24]. Since our prior review (2012) the sole use of fluoroquinolones as primary treatment for bacterial keratitis has increased alongside elevation in fluoroquinolone resistance [24]. Moreover, CoNS and Staphylococcus aureus have shown resistance to cephalotin and fluoroquinolones with the degree of resistance differing around the world [3]. Watson et al. (2018) demonstrated at the Sydney Eye Hospital that CoNS resistance was 9% to ciprofloxacin, cefatolin and gentamicin. Whereas Ni et al. (2015) found that resistance at the Wills Eye Hospital in Philadelphia was 32% to fluoroquinolones. Therefore, the inappropriate use of antibiotics may lead to antimicrobial resistance, prolonged recovery and poor outcomes in bacterial keratitis [3, 24]
3.2. Fungal Keratitis
The aetiology of microbial keratitis was found to differ depending on the geographical location of the patient and the infective pathogen [11]. Fungal keratitis is comparatively uncommon in temperate climates, though in tropical areas it can constitute a significant proportion of microbial keratitis [2, 13, 165]. The tropical climate, combined with greater agricultural and vegetation exposure in lower socioeconomic countries causes an increased risk of contracting fungal keratitis in populations [166, 167]. Moreover, similar to Karsten, Watson & Foster (2012), the fungal pathogens published in the literature were more often cultured from patients in these areas where they were more commonly exposed to contaminated soils and decaying vegetation while suffering ocular trauma [36, 168-170].
Fungi within the classification Mycota were the most common to cause keratitis; of the 83 families identified, only 3 did not belong to this classification (Table 2). Filamentous fungi, consistent with Karsten, Watson & Foster (2012), were found to be the most common cause of mycotic keratitis with Fusarium and Aspergillus species being the most prevalent [165, 171-173] and diverse genera with 38 and 25 species identified, respectively (Appendix 2).
Mycotic keratitis caused by filamentous fungi can involve any area of the cornea and often exhibits grey or yellow-white stromal infiltrate with indistinct margins [7, 174]. Progressive infiltration with multiple granular satellite stromal infiltrates is common and an immune-mediated ring infiltrate around the ulcer is often seen [7]. Yeasts may have a similar clinical presentation to filamentous fungi, however, yeasts tend to cause keratitis during immunosuppression and with systemic diseases such as diabetes, Human Immunodeficiency Virus (HIV), and corticosteroid therapy [174, 175].
The treatment of fungal keratitis consists of topical and systemic anti-fungal therapies [2, 176]. Natamycin 5% is often first line, although its effectiveness is limited by its poor penetration into the corneal stroma. Amphotericin B 0.3-0.5% is an alternative topical therapy but exhibits ocular toxicity [2]. Voriconazole is a proposed third option, however, the ‘Mycotic Ulcer Treatment Trial I’ found that voriconazole was inferior to natamycin in the treatment of all fungal keratitis, especially Fusarium keratitis [2, 176]. Since our prior review, the Mycotic Ulcer Treatment Trial II demonstrated that there was no therapeutic benefit of adding oral voriconazole to topical antifungal therapy [177]. Specifically, oral voriconazole, in addition to topical natamycin and voriconazole did not decrease the rate of corneal perforation nor the need for therapeutic penetrating keratoplasty. The study was halted due to the comparatively higher rates of corneal perforations in the oral voriconazole arm [177]. Filamentous fungi have been most frequently reported in our review, supporting current antifungal treatment recommendations [2, 178, 179] (Table 4).
3.3. Viral Keratitis
Viral pathogens are ubiquitous within populations, as evidenced by 90% of adults being seropositive for Herpes Simplex virus HSV antigens. However, as reported in the literature and Karsten, Watson & Foster (2012) only 20-30% of adults develop ocular manifestations from HSV infections [319]. Nonetheless, herpetic eye disease remains a significant cause of blindness worldwide, affecting over 1 million people annually [320].
The clinical features of HSV keratitis depend on the corneal layer affected and can typically include a dendritiform corneal ulcer or significant breakdown of the corneal epithelium with persistent punctate corneal keratopathy and corneal erosions [319]. Following the primary ocular infection, the viral genome may enter the surrounding nerve fibres and travel to the trigeminal nerve ganglion where it will remain in a latent state [319, 321]. Recurrent HSV infection can then occur in times of immunosuppression as the virus travels from the ganglion to infect the innervated tissue [319, 322]. Similar to HSV, the Varicella Zoster virus can remain dormant in the nerve ganglion following primary infection. Reactivation of the virus may lead to the development of Herpes Zoster Ophthalmicus [HZO], which presents as painful vesicular rash along the distribution of the innervation of the ophthalmic nerve [322]. Ocular manifestations of HZO can include Herpes Zoster keratitis [323].
From the review of the literature and consistent with Karsten, Watson & Foster (2012), Herpesviridae were the most common family implicated in viral keratitis. Herpes Simplex Virus Type 1 (HSV-1) was commonly responsible for viral keratitis affecting the skin and mucous membranes in the distribution of the trigeminal nerve [321]. Varicella Zoster virus, Cytomegalovirus, other members of the Herpesviridae family, were also implicated in viral keratitis, along with multiple species of Adenovirus (Table 5).
In the treatment of primary HSV epithelial keratitis acyclovir eye drops were the recommended therapy [17]. However, since Karsten, Watson & Foster (2012) publication, the Herpetic Eye Disease study II demonstrated that oral acyclovir may be utilised prophylactically to prevent recurrent herpetic keratitis, especially in patients with previous HSV stromal keratitis [319-324]. Furthermore, Bhatt et al. [2016], in their Cochrane Systematic review, demonstrated that oral acyclovir was beneficial in the prevention of recurrent herpes simplex keratitis in patients with corneal grafts [321]. Furthermore, the American Academy of Ophthalmology has produced guidelines to aid in the management of HSV Keratitis [325]. Herpes zoster keratitis is treated with oral antivirals, such as acyclovir or valacyclovir, in the first 72 hours to minimise the risk of ocular and other complications [17, 323].
3.4. Amoebic Keratitis
Amoebic pathogens are an uncommon cause of keratitis and typically occur in immunocompetent patients associated with ocular trauma and contact lens use [337]. Acanthamoeba, a unicellular protozoan, is a frequent cause of amoebic keratitis [338]. These protozoa exist in polluted soil or contaminated water supplies such as swimming pools, sewage and tap water [337, 339]. Acanthamoeba occur in both active trophozoite and dormant cyst forms. In favourable environments, the Acanthamoeba trophozoite exhibits high activity, although, in unfavourable environments such as during antibiotic and biocides treatment, Acanthamoeba from cysts with low activity but able to powerfully resist the surrounding environment [337, 338, 340].
The use of contact lenses, especially if used beyond recommended duration from the manufacturer and during contact with contaminated water, such as swimming, has been associated with a greater risk of Acanthamoeba keratitis [338, 339]. Similarly, ocular trauma with exposure to vegetable matter and contaminated soil heavily contributes to Acanthamoeba keratitis leading to a higher incidence in agricultural populations [167].
Similar to the study by Karsten, Watson & Foster (2012), Acanthamoeba was found to be the most common amoebic cause of keratitis in the current review [341]. Moreover, it was the most diverse, with 13 species of Acanthamoeba implicated as the cause of keratitis. Acanthamoeba is the most prevalent pathogenic amoeba, however, other families such as Vahlkampfia, Encephalotozoon, Vittaforma and Hartmanella have also been reported to cause amoebic keratitis (Table 6).
Acanthamoeba keratitis is often misdiagnosed as bacterial or mycotic keratitis, leading to inappropriate treatment and poor outcomes [257, 339]. Patients often present with pain that ranges from minimal to more commonly disproportionate levels for the clinical features shown [338]. Clinical features are often unilateral, including corneal epitheliopathy, stromal infiltrate, punctate keratopathy, ring infiltrate, perineural infiltrates and pseudo-dendrites [257]. As the disease progresses sterile anterior uveitis with a hypopyon may occur [338].
The prevention of Acanthamoeba keratitis prioritises the strict and appropriate use of contact lenses. Contact lenses must be cleaned and stored in appropriate disinfecting solutions and avoid exposure to potentially contaminated water supplies [338].
The current management of Acanthamoeba keratitis utilises a combination of anti-amoebic therapy, including a biguanide such as polyheramthylen biguanide 0.02%, or chlorhexideine 0.02% and a diamidine such as propamidine isethionate [339, 342]. It is imperative to institute appropriate therapy quickly to prevent Acanthamoeba penetrating deep into the cornea [338, 342]. However, due to the ability of Acanthamoeba to encyst, Acanthamoeba may remain dormant for extended periods before reactivation. Therefore, therapy combinations utilising diamidines are useful as these are effective against trophozoites and cysts [338, 340]. The use of corticosteroid therapy in Acanthamoeba keratitis is controversial, emerging evidence supports its use [343, 344], although there is no clear consensus [338, 345]. In cases of severe anterior segment inflammation, corticosteroids may be used, however, they must be used judiciously to prevent exacerbation of the infection and suppression of the host immune response [338].
| Family | Genus | Species | References |
|---|---|---|---|
| Adenoviridae | Adenovirus | - | [326, 327] |
| Adenovirus-3 | |||
| Adenovirus-8 | |||
| Adenovirus-19 | |||
| Herpesviridae | Cytomegalovirus | - | [328] |
| Lymphocryptovirus | Human herpesvirus 4 | [329] | |
| Simplexvirus | Herpes Simplex Virus-1 | [327, 330] | |
| Herpes Simplex Virus-2 | |||
| Varicellovirus | Varicella Zoster Virus | [331] | |
| Paramyxoviridae | Morbillivirus | Rubeola virus | [332] |
| Rubulavirus | Mumps rubulavirus | [333] | |
| Picornaviridae | Enterovirus | - | [334] |
| Enterovirus B | |||
| Poxviridae | Orthopoxvirus | Vaccinia Virus | [335, 336] |
| Family | Genus | Species | References |
|---|---|---|---|
| Acanthamoebidae | Acanthamoeba | Astronyxis | [341, 346-353] |
| Castellanii | |||
| Culbertsoni | |||
| Griffini | |||
| Hatchetti | |||
| Lenticulata | |||
| Lugdunesis | |||
| Mauritaniensis | |||
| Palestinensis | |||
| Polyphaga | |||
| Quina | |||
| Rhysodes | |||
| Triangularis | |||
| Demodicidae | Demodex | Brevis | [354] |
| Folliculorum | |||
| Hartmannellidae | Hartmanella | - | [355, 356] |
| Vermiformis | |||
| Nosematidae | Anncaliia | Algerae | [357] |
| Nosema | Corneum | [358] | |
| Vittaforma | Corneae | [359] | |
| Onchocercidae | Onchocerca | Volvulus | [142] |
| Tarsonemidae | Tarsonemid | Heterostigmae | [360] |
| Unikaryonidae | Encephalitozoon | Cuniculi | [359] |
| Hellem | |||
| Intestinalis | |||
| Valkampfiidae | Vahlkampfia | - | [361, 362] |
| Paravahlkampfia | - | [363] |
CONCLUSION
Microbial keratitis is an ophthalmic emergency as it can lead to irreversible damage to the cornea and loss of vision [1, 3, 5]. To minimise complications, prompt and effective treatment must be instituted to prevent permanent damage to the cornea. For the successful treatment of microbial keratitis, it is vital to correctly identify the causative organism as it enables the use of the most effective treatments to treat the infection [3].
A comprehensive and exhaustive review of the literature yielded 688 species representing 271 genera from 145 families being implicated in microbial keratitis, in contrast to the 232 species from 142 genera representing 80 families found by Karsten, Watson & Foster (2012). Similarly, from the current review of the literature, there were 135 microbial species found to cause keratitis published before 2012 that were not identified by Karsten, Watson & Foster (2012).
Similar to the study by Karsten, Watson & Foster (2012), the current literature demonstrated that bacterial keratitis was the most common and fungal pathogens were the most diverse, with 391 species identified as causative pathogens of microbial keratitis. Comparatively, there were 254 bacterial species, 27 species of amoeba and 14 viral species found to cause microbial keratitis (Fig 1).
Correspondingly, the geographical variances noted from Karsten, Watson & Foster (2012) were further reinforced from the literature. It was found that bacterial keratitis is relatively common in developed nations, likely due to the use of contact lenses [14]. Similarly, fungal keratitis is more common in tropical environments and the developing world, most likely due to greater exposure to decaying vegetation and soil [165, 167]. Furthermore, many of the pathogens reported in the literature were often from studies and case reports in patients that had been exposed to poor sanitation, greater agricultural exposure and ocular trauma leading to the development of keratitis [170, 182, 251, 265, 311].
Similarly, the treatment of microbial keratitis has remained similar since Karsten, Watson & Foster (2012). Bacterial keratitis treatment commonly consists of topical fluoroquinolones, yet there is an increasing risk of antimicrobial resistance [24], necessitating the need for judicious and appropriate use of antimicrobials [3]. Contrastingly, since Karsten, Watson & Foster (2012), available evidence now suggests that oral antifungals, in addition to topical therapy do not add a therapeutic benefit [177].
This review has demonstrated the great diversity of the pathogens implicated in microbial keratitis. Relative to Karsten, Watson & Foster (2012), further 443 pathogens were identified as causative organisms of microbial keratitis. Additionally, there has been further investigation of antimicrobial resistance in bacterial keratitis and changes in the management of microbial keratitis. Therefore, the current review provides an important update on the spectrum and management of microbial keratitis, to assist clinicians in its diagnosis and treatment and help reduce the associated morbidity. Advancing the literature into the epidemiology and geographical variance of causative organisms will further aid in the identification and treatment of microbial keratitis.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
APPENDIX
| Family | Genus | Species | References |
|---|---|---|---|
| Fungal Keratitis | |||
| Ajellomycetaceae | Blastomyces | dermatitidis | [171] |
| Histoplasma | capsulatum | [180] | |
| Paracoccidioides | brasiliensis | [175] | |
| Amphisphaeriaceae | Pestalotiopsis | clavispora | [182] |
| Arthrodermataceae | Epidermophyton | floccosum | [181] |
| Microsporum | - | [175, 228, 364] | |
| canis | |||
| gypseum | |||
| Trichophyton | - | [171, 183-185] | |
| capitatum | |||
| mentagrophytes | |||
| schoenleinii | |||
| verrucosum | |||
| Ascodesmiaceae | Cephaliophora | - | [186, 196] |
| irregularis | |||
| Basidiobolaceae | Basidiobolus | ranarum | [187] |
| Bionectriaceae | Gliocladium | - | [186] |
| Botryosphaeriaceae | Auerswaldia | lignicola | [188] |
| Botryosphaeria | - | [169, 201] | |
| dothidea | |||
| rhodina | |||
| Diplodia | - | [11] | |
| Lasiodiplodia | theobromae | [196, 207] | |
| Macrophomina | phaseolina | [169, 208] | |
| Neoscytalidium | oculus | [203] | |
| Neofusicoccum | mangiferae | [209] | |
| Sphaeropsis | subglobosa | [175] | |
| Cephalothecaceae | Phialemonium | - | [202, 210] |
| curvatum | |||
| Ceratobsidiaceae | Rhizoctonia | - | [169, 175] |
| bataticola | |||
| Chaetomiaceae | Chaetomium | atrobrunneum | [47, 168, 191, 214] |
| globosum | |||
| strumarium | |||
| Humicola | - | [12] | |
| Thielavia | heterothallica | [169, 204, 205] | |
| subthermophila | |||
| tortuosa | |||
| Chaetosphaerellaceae | Diplosporium | - | [175] |
| Chaetosphaeriaceae | Gongromeriza | - | [186] |
| Trichothecium | - | [186, 206] | |
| Clavicipitaceae | Metarhizium | - | [222-224] |
| anisopliae | |||
| Coniochaetaceae | Lecythophora | - | [175, 210] |
| mutabilis | |||
| Cordycipitaceae | Acrostalagmus | cinnabarensis | [28] |
| Beauveria | - | [47, 191, 229, 230] | |
| bassiana | |||
| Engyodontium | alba | [175] | |
| Corticiaceae | Sporotrichum | schenekii | [28, 233] |
| Corynesporascaceae | Corynespora | cassiicola | [251] |
| Cryptococcaceae | Cryptococcus | - | [212, 222, 252, 253] |
| albidus | |||
| curvatum | |||
| laurentii | |||
| neoformans | |||
| Cunninghamellaceae | Cunninghamella | - | [186, 254] |
| spinosum | |||
| Cystofilobasidiaceae | Guehomyces | pullulans | [222] |
| Davidiellaceae | Cladosporium | - | [115, 247, 257] |
| Davidiella | tassiana | [204] | |
| Debaryomycetaceae | Meyerozyma | caribbica | [259] |
| Dermateaceae | Gloeosporium | fructigenum | [261] |
| Diaporthaceae | Phomopsis | - | [262, 263] |
| phoenicicola | |||
| Didymellaceae | Epicoccum | - | [12, 191, 266] |
| nigrum | |||
| sorghi | |||
| Phoma | - | [12, 92, 175] | |
| eupyrena | |||
| oculo-hominis | |||
| Dipodascaceae | Geotrichum | - | [175, 210, 281] |
| candidum | |||
| Magnusiomyces | capitatus | [282] | |
| Dothioraceae | Aureobasidium | - | [12, 189, 207, 283] |
| pullulans | |||
| Eremomycetaceae | Arthrographis | kalrae | [175, 284] |
| Gjaerumiaceae | Gjaerumia | minor | [285] |
| Helotailes incertae sedis | Scytalidium | dimidiatum | [187, 287] |
| hyalinum | |||
| Herpotrichiellaceae | Cladophialophora | bantiana | [196, 240, 293] |
| cladosporioides | |||
| carrionii | |||
| Exophiala1 | dermatitidis | [115, 146, 154, 175, 289-292] | |
| jeanselmei | |||
| jeanselmei var. dermatitidis | |||
| jeanselmei var. jeanselmei | |||
| moniliae | |||
| phaeomuriformis | |||
| spinifera | |||
| Fonsecaea | compacta | [47, 115, 191, 294] | |
| pedrosoi | |||
| Phialophora | bubakii | [173, 175, 283] | |
| pedrosoi | |||
| verrucosa | |||
| Pullularia | - | [206] | |
| Torula | - | [295] | |
| Hypocreaceae | Acremonium | - | [127, 166, 175, 191, 193, 247, 296] |
| atrogriseum | |||
| curvum | |||
| falciforme | |||
| kiliense | |||
| potronii | |||
| recifei | |||
| strictum | |||
| Acrostalagmus | cinnabarensis | [28] | |
| Gliocladium | - | [186] | |
| Sepedonium | - | [270, 283] | |
| Trichoderma | - | [28, 146, 193, 222, 240] | |
| hamatum | |||
| longibrachiatum | |||
| koningii | |||
| Hypocreales Incertae sedis | Cephalosporium | - | [102, 146] |
| Myrothecium | - | [175] | |
| Hyponectriaceae | Humicola | - | [12] |
| Glomerellaceae | Colletotrichum | - | [41, 189-195] |
| atramentum | |||
| capsici | |||
| coccodes | |||
| dematium | |||
| gleosporiodes | |||
| graminicola | |||
| truncatum | |||
| Glomerella | cingulata | [78, 169] | |
| Gymnoascaceae | Gymnoascus | - | [197] |
| Lagenidiaceae | Lagenidium | - | [198] |
| Lasiosphaeriaceae | Arthrinium | phaeospermum | [173, 193] |
| Cladorrhinum | bulbilosum | [199, 200] | |
| Monotospora | - | [186] | |
| Podospora | - | [169] | |
| Lichtheimiaceae | Lichtheimia | corymbifera | [193, 204] |
| ramosa | |||
| Lophiostomataceae | Tetrapola | - | [211] |
| Malasseziaceae | Malassezia | - | [175, 212, 213] |
| furfur | |||
| restricta | |||
| Massarinaceae | Helminthosporium | - | [10, 90] |
| maydis | |||
| Metacapnodiaceae | Hormiscium | - | [186] |
| Microascaceae | Doratomyces | - | [186] |
| Cephalotrichum | stemonitis | [175] | |
| Graphium | eumorphum | [216] | |
| Lophotrichus | - | [217] | |
| Microascus | brevicaulis | [29, 191, 218] | |
| gracilis | |||
| Monosporium | - | [219] | |
| Pseudallescheria | boydii | [47, 191] | |
| Scedosporium | apiospermum | [174, 196, 220, 221] | |
| boydii | |||
| prolificans | |||
| Scopularisopsis | - | [28, 186, 189, 215] | |
| blochi | |||
| brevicaulis | |||
| Wardomyces | - | [212] | |
| Montagnulaceae | Microsphaeropsis | olivacea | [225] |
| Mucoraceae | Chlamydoabsidia | padenii | [175] |
| Mucor | - | [10, 28, 226, 228] | |
| cornealis | |||
| racemosus | |||
| ramosissimus | |||
| Rhizomucor | - | [29] | |
| Rhizopus | arrhizus | [28, 173, 191, 193, 219, 226, 227] | |
| nigricans | |||
| oryzae | |||
| parasiticus | |||
| stolonifer | |||
| Zygorhynchus | - | [186] | |
| Mycosphaerellaceae | Cercospora | - | [186] |
| Hormodendrum | - | [227, 231] | |
| Microcyclosporella | mali | [232] | |
| Nectriaceae | Bactridium | - | [186] |
| Cylindrocarpon | - | [11, 191, 247, 365] | |
| destructans | |||
| lichenicola | |||
| Fusarium | - | [127, 146, 172, 175, 193, 199, 234-246] | |
| aquaeductum | |||
| asiaticum | |||
| boothii | |||
| cerealis | |||
| chlamydosporum | |||
| culmorum | |||
| delphinoides | |||
| dimerum | |||
| episphaeria | |||
| equiseti | |||
| graminearum | |||
| incarnatum | |||
| incarnatum-equiseti | |||
| keratoplasticum | |||
| langsethiae | |||
| lateritium | |||
| lichenicola | |||
| moniliforme | |||
| musae | |||
| napiforme | |||
| nivale | |||
| nygamai | |||
| Nectriaceae | Fusarium | oxysporum | [127, 146, 172, 175, 193, 199, 234-246] |
| penzigii | |||
| poae | |||
| polyphialidicum | |||
| proliferatum | |||
| pseudograminearum | |||
| roseum | |||
| sacchari | |||
| sambucinum | |||
| solani | |||
| sporotrichioides | |||
| subglutinans | |||
| temperatum | |||
| tricinctum | |||
| ventricosum | |||
| verticilloides | |||
| Fusidium | terricola | [28] | |
| Fusoma | - | [186] | |
| Gibberalla | avenacea 2 | [173, 186, 193] | |
| fujikuroi 3 | |||
| Moniliaceae | - | [238] | |
| Neocosmospora | keratoplastica | [204, 248, 249] | |
| rubicola | |||
| vasinfecta | |||
| Sarcopodium | oculorum | [250] | |
| Volutella | - | [112] | |
| Niessliaceae | Stachybotrys | - | [186] |
| Onygenaceae | Chrysosporium | - | [29, 47, 171, 175] |
| inops | |||
| parvum | |||
| Coccidioides | immitis | [175] | |
| Ophiocordycipitaceae | Purpureocillium | lilacinum | [255] |
| Ophiostomataceae | Sporothrix | pallida | [228, 256] |
| schenckii | |||
| Orbiliaceae | Arthrobotrys | oligospora | [258] |
| Phaeosphaeriaceae | Tintelnotia | destructans | [260] |
| Phycomycetaceae | Periconia | keratidis | [28] |
| Plectosphaerellaceae | Plectosporium | tabacinum | [265] |
| Verticillium | - | [175, 264] | |
| searrae | |||
| Pleosporaceae | Alternaria | alternata | [171, 191, 204, 270-272] |
| chlamydospora | |||
| fusispora | |||
| infectoria | |||
| longipes | |||
| nees | |||
| tenuissima | |||
| Bipolaris | - | [146, 196, 257, 273, 274, 283] | |
| australiensis | |||
| hawaiiensis | |||
| oryzae | |||
| Pleosporaceae | sorokiniana | ||
| spicifera | |||
| Brachysporium | - | [275] | |
| Cochliobolus | - | [169, 240] | |
| heterostrophus | |||
| spicifer | |||
| Curvularia | borreriae | [34, 111, 115, 171, 175, 191, 249, 267-269] | |
| brachyspora | |||
| clavata | |||
| crepinii | |||
| fallax | |||
| geniculata | |||
| lunata | |||
| pallescens | |||
| prasadii | |||
| senegalensis | |||
| spicifera | |||
| verruculosa | |||
| Dichotomophthoropsis | nymphaearum | [249] | |
| Drechslera | - | [220, 269] | |
| halodes | |||
| Edenia | gomezpompae | [193] | |
| Exserohilum | - | [175, 190, 199, 207, 276] | |
| longirostratum | |||
| mcginnisii | |||
| roseum | |||
| rostratum | |||
| solani | |||
| Pithomyces | - | [34, 212] | |
| Pleospora | tarda | [146, 240] | |
| Pyrenochaeta | - | [277, 278] | |
| keratinophila | |||
| Stemphylium | - | [279] | |
| Ulocladium | atrum | [280] | |
| Pleurotheciaceae | Phaeoisaria | clematidis | [79] |
| Polyporaceae | Trametes | betulina | [80] |
| Pythiaceae | Pythium | insidiosum | [47, 81] |
| Saccharomycetaceae | Blastoschizomyces | capitatus | [175] |
| Candida | albicans | [82-86, 105, 170, 191, 212, 222, 236, 259, 286] | |
| ciferrii | |||
| curvata | |||
| dubliniensis | |||
| famata | |||
| fermentati | |||
| glabrata | |||
| guilliermondii | |||
| krusei4 | |||
| lusitaniae | |||
| lypolytica | |||
| orthopsilosis | |||
| parapsilosis | |||
| pelliculosa | [18, 48, 53, 61, 68, 125, 163-169] | ||
| rugosa | |||
| tropicalis | |||
| utilis | |||
| viswanathii | |||
| zeylanoides | |||
| Saccharomyces | cerevisiae | [9] | |
| Schizophyllaceae | Schizophyllum | commune | [288] |
| Schizoporaceae | Hyphodontia | - | [201] |
| Sclerotiniaceae | Botrytis | - | [28] |
| Sebacinaceae | Piriformospora | - | [146] |
| Septobasidiaceae | Glenospora | graphii | [175] |
| Sordariales incertae sedis | Papulaspora | - | [191, 199] |
| equi | |||
| Sordariaceae | Neurospora | - | [47, 146] |
| sitophila | |||
| Sporidiobolaceae | Rhodotorula | - | [270, 283, 308-311] |
| glutinis | |||
| minuta | |||
| mucilaginosa | |||
| Sporocadaceae | Pseudopestalotiopsis | theae | [313] |
| Sordariales | Pleurothecium | recurvatum | [315] |
| Sympoventuriaceae | Verruconis | gallopava | [77] |
| Syncephalastraceae | Syncephalastrum | - | [189] |
| Togniniaceae | Phaeoacremonium | - | [316, 317] |
| parasiticum | |||
| Torulaceae | Torula | - | [12] |
| Tetraplosphaeriaceae | Tetraploa | - | [102, 175] |
| aristata | |||
| Tremellaceae | Bulleromyces | - | [212] |
| Trichocomaceae | Aspergillus | alternata | [41, 115, 146, 171, 175, 191, 196, 199, 210, 236, 247, 297-302] |
| brasiliensis | |||
| cibarius | |||
| clavatus | |||
| fischerianus | |||
| flavipes | |||
| flavus | |||
| fumigatus | |||
| glaucus | |||
| janus | |||
| japonicus | |||
| nidulans | |||
| niger | |||
| niveus | |||
| nominus | |||
| ochraceus | |||
| oryzae | |||
| pseudotamarii | |||
| sydowii | |||
| tamarii | |||
| terreus | |||
| tubingensis | |||
| versicolour | |||
| viridinutans | |||
| wentii | |||
| Neosartorya | udagawae | [303] | |
| Penicillium | brocae | [9, 102, 146, 175, 210, 240, 306, 307] | |
| canescens | |||
| chrysogenum | |||
| citrinum | |||
| crustaceum | |||
| expansum | |||
| implicatum | |||
| marneffei | |||
| notatum | |||
| piceum | |||
| spinulosum | |||
| Paecilomyces | - | [95, 154, 270, 305] | |
| farinosus | |||
| lilacinus | |||
| variotti | |||
| Sagenomella | keratitidis | [304] | |
| Trichosphaeriaceae | Khuskia | - | [312] |
| Nigrospora | - | [12, 191, 283] | |
| sphaerica | |||
| Trichosporonaceae | Trichosporon | anisopliae | [78, 105, 127, 309, 312, 314] |
| asahii | |||
| beigelii | |||
| capitatum | |||
| mucoides | |||
| rugosum | |||
| Tritirachiaceae | Tritirachium | oryzae | [175] |
| Ustilaginaceae | Rhodosporidium | toruloides | [175] |
| Ustilago | - | [175] | |
| Venturiaceae | Fusicladium | - | [186] |
| Wickerhamomycetaceae | Cyberlindnera | fabianii | [259] |
| Wickerhamomyces | anomalus | [318] | |
| n/a | Botryodiplodia | - | [366] |
| Dichotomophthoropsis | - | [175, 312] | |
| nymphearum | |||
| portulacae | |||
| Mycelia | - | [314, 367] | |
| sterilia | |||
| Ovadendron | sulphureo-ochraceum | [175] | |
| Phaeotrichoconis | crotalariae | [175] | |
| keratinophila | |||
Table 2.
| Family | Genus | Species | References |
|---|---|---|---|
| Bacterial Keratitis | |||
| Acetobacteraceae | Roseomonas | - | [25] |
| Actinomycetaceae | Actinomyces | bovis | [27, 28] |
| israelii | |||
| Aerococcaceae | Abiotrophia | defectiva | [31] |
| Aerococcus | - | [32, 33] | |
| viridans | |||
| Aeromonadaceae | Aeromonas | - | [15, 41, 42] |
| hydrophilia | |||
| Alcaligenaceae | Achromobacter | - | [33, 42, 51, 52] |
| denitrificans | |||
| xylosoxidans | |||
| Alcaligenes | faecalis | [12] | |
| Bacillaceae | Bacillus | cefilius | [60-64] |
| cereus | |||
| circulans | |||
| coagulans | |||
| firmus | |||
| licheniformis | |||
| megaterium | |||
| polymyxa | |||
| subtilis | |||
| Bacteroidaceae | Bacteroides | fragilis | [68] |
| Bartonellaceae | Bartonella | henselae | [87] |
| Brevibacteriaceae | Brevibacterium | - | [12] |
| Brucellaceae | Ochrobactrum | anthropi | [93] |
| Pasteurella | canis | [49, 94] | |
| multocida | |||
| Burkholderiaceae | Burkholderia | ambifaria | [47, 96, 97] |
| cepacia | |||
| gladioli | |||
| Cardiobacteriaceae | Suttonella | indologenes | [34] |
| Carnobacteriaceae | Dolosigranulum | pigrum | [101] |
| Caulobacteraceae | Brevundimonas | diminuta | [108] |
| Clostridiaceae | Clostridium | - | [60, 110] |
| perfringens | |||
| Peptostreptococcus | anaerobius | [68, 111] | |
| micros | |||
| Sarcina | - | [112] | |
| Comamonadaceae | Delftia1 | acidovorans | [55, 98] |
| Corynebacteriaceae | Corynebacterium | accolens | [15, 44, 62, 105, 115-121] |
| bovis | |||
| diptheriae | |||
| hofmannii | |||
| macginleyi | |||
| matruchotii | |||
| minutissimum | |||
| propinquum | |||
| pseudodiphtheriticum | |||
| pyogenes | |||
| striatum | |||
| urealyticum | |||
| ureicelerivorans | |||
| xerosis | |||
| Arcanobacterium | haemolyticum | [117] | |
| Enterobacteriaceae | Citrobacter | diversus | [43, 105, 122, 123] |
| koseri | |||
| freundii | |||
| Enterobacter | aerogenes | [34, 61, 123, 146] | |
| cloacae | |||
| gergoviae | |||
| Escherichia | coli | [52, 124] | |
| Hafnia | alvei | [125] | |
| Klebsiella | ornithinolytica | [105, 117, 126, 127, 146] | |
| oxygenate | |||
| oxytoca | |||
| ozaenae | |||
| pneumoniae | |||
| Morganella | morganii | [368] | |
| Pantoea | - | [65, 129] | |
| agglomerans | |||
| Proteus | - | [52, 126, 130, 368] | |
| mirabilis | |||
| rettgeri | |||
| vulgaris | |||
| Providencia | - | [33, 104, 131-133] | |
| alcalifaciens | |||
| rettgeri | |||
| stuartii | |||
| Raoultella | - | [33, 134] | |
| ornithinolytica | |||
| Salmonella | - | [102] | |
| Serratia | liguefaciens | [52, 135] | |
| marcescens | |||
| Shigella | flexneri | [52, 136] | |
| sonnei | |||
| Enterococcaceae | Enterococcus | faecalis | [33, 39, 138] |
| faecium | |||
| Eubacteriaceae | Eubacterium | aerofaciens | [143] |
| Flavobacteriaceae | Capnocytophaga | - | [147-151] |
| canimorsus | |||
| cynodegmi | |||
| ochracea | |||
| sputigena | |||
| Chryseobacterium | - | [152-154] | |
| indologenes | |||
| Elizabethkingia | meningoseptica | [34, 155] | |
| Flavobacterium | - | [52] | |
| Fusobacteriaceae | Fusobacterium | - | [157] |
| Intrasporangiaceae | Ornithinimicrobium | pekingense | [160] |
| Lactobacillaceae | Lactobacillus | - | [162] |
| Leuconostocaceae | Leuconostoc | mesenteroides | [10] |
| Listeriaceae | Listeria | monocytogenes | [26] |
| Microbacteriaceae | Microbacterium | - | [29, 30] |
| oxydans | |||
| Micrococcus | - | [10, 30, 38, 39] | |
| luteus | |||
| Micrococcaceae | tetragenus | ||
| Kocuria | koreensis | [10, 34-37] | |
| kristinae | |||
| palustris | |||
| rosea | |||
| varians | |||
| Rothia | dentocariosa | [35, 40] | |
| mucilaginosa | |||
| Moraxellaceae | Acinetobacter | baumannii | [43-48] |
| calcoaceticus | |||
| calcoaceticus var. antitratus | |||
| haemolyticus | |||
| lwoffi | |||
| junii | |||
| schindleri | |||
| Moraxella | catarrhalis | [30, 49, 50] | |
| lacunata | |||
| nonliquefaciens | |||
| Mycobacteriaceae | Mycobacterium | abscessus | [47, 53-59] |
| asiaticum | |||
| aurum | |||
| avium | |||
| chelonae | |||
| flavescens | |||
| fotuitum | |||
| gordonae | |||
| intracellulare | |||
| marinum | |||
| massiliense | |||
| mucogenicum | |||
| nonchromogenicum | |||
| szulgai | |||
| terrae | |||
| triviale | |||
| Neisseriaceae | Eikenella | corrodens | [51] |
| Kingella | denitrificans | [51, 67] | |
| kingae | |||
| Neisseria | gonorrhoea | [13, 65, 66] | |
| meningitides | |||
| mucosa | |||
| Nocardiaceae | Nocardia | abscessus | [44, 69-76] |
| actinomycetes | |||
| amamiensis | |||
| araoensis | |||
| arthritidis | |||
| asiatica | |||
| asteroides | |||
| beijingensis | |||
| blacklockiae | |||
| brasiliensis | |||
| carnea | |||
| caviae | |||
| cyriacigeorgica | |||
| Nocardia | elegans | [237, 291-298] | |
| farcinica | |||
| ignorata | |||
| levis | |||
| neocaledoniensis | |||
| nova | |||
| otitidiscaviarum | |||
| pneumoniae | |||
| pseudobrasiliensis | |||
| puris | |||
| rhamnosiphila | |||
| thailandica | |||
| transvalensis | |||
| wallacei | |||
| Rhodococcus | equi | [77] | |
| Pasteurellaceae | Aggregatibacter | actinomycetemcomitans | [88, 89] |
| aphrophilus | |||
| Haemophilus | aegyticus | [41, 44, 46, 90, 91] | |
| haemoglobinophilus | |||
| influenza | |||
| parainfluenza | |||
| Peptococcaceae | Peptococcus | prevotii | [92] |
| Porphyromonadaceae | Parabacteroides | distasonis | [95] |
| Propionibacteriaceae | Propionibacterium | - | [1, 68, 98, 99] |
| acnes | |||
| granulosum | |||
| Prevotellaceae | Prevotella | - | [68, 100] |
| intermedia | |||
| melaninogenica | |||
| Pseudomonadaceae | Azotobacter | beijerinckii | [107] |
| chroococcum | |||
| paspali | |||
| vinelandii | |||
| Pseudomonas | acidovorans | [30, 33, 38, 52, 102-106] | |
| aeruginosa | |||
| fluorescens | |||
| luteola | |||
| mesophilic | |||
| oryzihabitans | |||
| putida | |||
| pyocyaneus | |||
| stutzeri | |||
| Rhizobiaceae | Rhizobium | radiobacter | [109] |
| Rhodobacteraceae | Paracoccus | yeei | [113] |
| Rickettsiaceae | Rickettsia | conorii | [114] |
| Sphingobacteriaceae | Sphingobacterium | spiritivorum | [30] |
| Spirochaetaceae | Borrelia | burgdorferi | [369] |
| Treponema | pallidum | [137] | |
| Gemella | - | [33, 38, 370] | |
| haemolysans | |||
| morbillorum | |||
| Staphylococcaceae | Staphylococcus | albus | [10, 15, 23, 34, 39, 43, 46, 52, 60, 139-142] |
| aureus | |||
| auricularis | |||
| capitis | |||
| caprae | |||
| cohnii | |||
| epidermidis | |||
| haemolyticus | |||
| hominis | |||
| hyicus | |||
| Staphylococcaceae | Staphylococcus | intermedius | [10, 15, 23, 34, 39, 43, 46, 52, 60, 139-142] |
| lentus | |||
| lugdunensis | |||
| MRSA | |||
| pasteuri | |||
| saprophyticus | |||
| simulans | |||
| warneri | |||
| xylosus | |||
| Staphylococcaceae | Staphylococcus | acidominimus | [33, 41, 46, 47, 49, 60, 64, 65, 117, 126, 144-146] |
| agalactiae | |||
| anginosus | |||
| dysgalactiae | |||
| intermedius | |||
| mitis | |||
| morbillorum | |||
| oralis | |||
| parasanguis | |||
| pneumoniae | |||
| pyogenes | |||
| salivarius | |||
| sanguinis | |||
| viridans | |||
| Streptomycetaceae | Streptomyces | - | [131, 156] |
| thermocarboxydus | |||
| Tsukamurellaceae | Tsukamurella | hongkongensis | [158, 159] |
| spumae | |||
| tyrosinosolvens | |||
| Vibrionaceae | Vibrio | vulnificus | [43, 161] |
| parahaemolyticus | |||
| Xanthomonadaceae | Stenotrophomonas | maltophilia | [46, 98] |
| Xanthomonas | - | [163] | |
| Yersiniaceae | Yersinia | - | [43, 164] |
| pseudotuberculosis | |||


