All published articles of this journal are available on ScienceDirect.
Repeatability and Reproducibility of Tear Film Evaporation Rate Measurement using a new Closed-Chamber Evaporimeter
Abstract
Purpose:
This study evaluates the repeatability and reproducibility of tear film evaporation rate measurement using a commercially available handheld closed-chamber evaporimeter (VapoMeter, Delfin Technologies, Finland).
Study Design:
This was a randomized observational study, in which two visits were required. At visit 1, screening tests were performed on the participants. Subsequently, tear evaporation was measured thrice by examiner 1 (E1). The procedure was then repeated by examiner 2 (E2) at visit 2.
Methods:
40 healthy participants with no ocular diseases were recruited for this study. A closed chamber evaporimeter was used in this study (VapoMeter, Delfin Technologies, Finland). Primary investigations, including slit-lamp examination, tear production test, and ocular discomfort, were performed during the first visit for the purpose of screening.
Results:
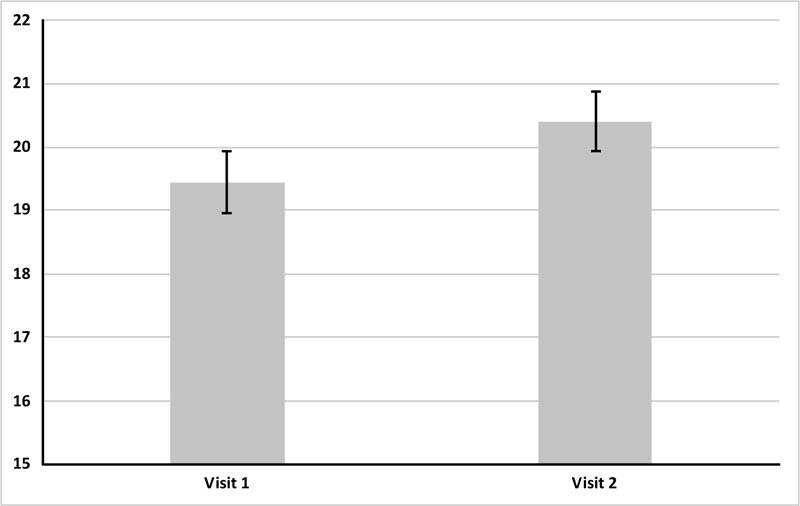
The mean of the three measurements of tear evaporation obtained by examiner 1 at visit 1 was 19.38 ± 0.79 g/m2/h, and the mean of the three readings obtained by examiner 2 at visit 2 was 20.49 ± 0.48 g/m2/h. The average Intraclass Correlation Coefficient (ICC) among the three readings of tear evaporation was 0.84 and 0.63 with a 95% confidence interval (CI) at visits 1 and 2, respectively. A comparison of the reliability of the measurements from the two examiners revealed an ICC of 0.69 with a 95% CI.
Conclusion:
The VapoMeter provides repeatable and reproducible measurements of tear film evaporation. This study demonstrates that the VapoMeter could provide clinicians with a readily available method for rapid evaluation of tear film evaporation. By considering the significance of tear evaporation as a diagnostic tool for dry eyes, the VapoMeter may help to diagnose better and manage dry eye syndrome.
1. INTRODUCTION
Evaporative dry eye is one of the main classification groups of symptomatic dry eye syndrome [1]. Evaporative dry eye can be caused by many factors, including meibomian lipid deficiency, low blink rate, lid aperture, or dynamics disorders. Furthermore, extrinsic factors, including wearing contact lenses or exposure to adverse environmental factors, could cause evaporative dry eye [1, 2].
The lipid layer plays an important role in tear film stability. The nonpolar phase of the lipid layer is responsible for controlling the water vapor evaporation from the aqueous layer [3]. However, chronic ocular diseases, such as blepharitis, may lead to meibomian gland dysfunction and affect lipid secretion, thereby altering the lipid layer of the tear film [4, 5]. Several diagnostic tools have been proposed to clinically diagnose and monitor dry eye syndrome. One of these diagnostic methods is evaluating the evaporation rate of the tear film. In this technique, the average water loss from the exposed ocular surface is measured while the eye is open [6].
Different clinical techniques have been used to assess tear film evaporation since the first studies by Hamano et al. and Rolando et al. in the 1980s [7, 8]. In the 1990s, a new method using the principle of measuring vapor pressure produced by relative humidity between two points was used by Yamada and Tsubota [9]. In this method, a moisture sensor was inserted into a chamber covering the eye [9, 10]. Later, Tress and Tomlinson have developed a new humidity evaluation unit using the same principle [11]. Here, a Servomed evaporimeter was modified by attaching swimming goggles and linking them to a computer for the analysis and storage of data [11, 12].
However, the benefits of these methods were overweighed by many drawbacks, including complicated setup and modification processes, requiring many instruments to be connected together to measure, analyze, and store data. Because of these design issues, these instruments are not portable and commercially available. Furthermore, they need longer testing times to measure tear evaporation than new techniques that take a few seconds to provide the final evaporation values. These drawbacks are the leading cause for the inability of these techniques to be used clinically and the unavailability of a commercial device for clinically evaluating the tear film evaporation rate. For overcoming these disadvantages, a novel noncontact technique using infrared thermographic imaging of the ocular surface has been proposed [13, 14]. However, further studies on this methodological approach are needed to validate and assess its accuracy.
Transepidermal water loss is an essential parameter used to assess skin barrier function [15]. The VapoMeter (Delfin Technologies, Kuopio, Finland), a closed-chamber evaporimeter, is a commonly used device for evaluating this transepidermal water loss in dermatology clinics [16, 17]. In this study, we used a modified, commercially available dermatology device to measure the tear film evaporation rate. This study evaluated the repeatability and reproducibility of tear film evaporation rate measurement using the VapoMeter, a commercially available closed-chamber evaporimeter.
2. METHODS
2.1. Participants
40 healthy participants without ocular diseases were recruited for this study (mean age, 22.39 ± 2.33 years). Ethical approval was obtained from the College of Applied Medical Science Research Center, King Saud University. The study was conducted according to the ethical principles enshrined in the Declaration of Helsinki. Informed consent was obtained from all participants after explaining the study procedure.
2.2. Screening Investigations
Primary investigations, including slit-lamp examination, tear production test, and ocular discomfort, were performed during the first visit for screening. Tear production was evaluated using the phenol red thread test (PRT), and the Ocular Surface Disease Index (OSDI) questionnaire was used to assess ocular discomfort. Participants with any ocular disease and those who were contact lens wearers were excluded from this study.
2.3. Tear Evaporation Test
The VapoMeter, a closed-chamber evaporimeter, was used in this study. This is a handheld portable device used for measuring transepidermal water loss. The device was modified by attaching swimming goggles to measure the evaporation rate of the eye and surrounding skin [18]. The device consists of two sensors that monitor changes in relative humidity and temperature inside the chamber. The evaporation rate (g/m2/ h) is automatically calculated and displayed on the device screen.
2.4. Clinical Procedures
The study protocol required examinations at two visits. All tear evaporation evaluations were performed after 11 am to avoid possible variations in measurements in which the relative humidity and ambient temperature of the examination room were monitored. At visit 1, screening tests were performed on all participants. Then, three tear evaporation measurements were obtained by examiner 1 (E1). The same procedure was performed by examiner 2 (E2) at visit 2.
Noninvasive measurements of the evaporation rate were obtained by placing the VapoMeter over the participants’ eyes. The measurements were obtained with the eyes open and closed to account for evaporation from the surrounding skin. During open-eye measurements, the participants were instructed to look straight and not blink during the test.
2.5. Statistical Analysis
The data were analyzed using Statistical Package for the Social Sciences, version 22 (IBM Corp., Armonk, NY, USA). The normality of data distribution was tested using the Kolmogorov–Smirnov test. Repeated-measures analysis of variance was performed for normally distributed data whereas non-normally distributed data were analyzed using the Friedman test and post-hoc Wilcoxon rank-sum test. The intraclass correlation coefficient (ICC) was calculated to assess the inter-rater and intra-rater reliability of the data collected at each visit. The correlation between parameters was assessed by calculating Pearson correlation and Spearman rank-order correlation coefficients for the normally and non-normally distributed data, respectively.
3. RESULTS
40 participants were enrolled in this study (40 men: mean age, 22.39 ± 2.33 years). The mean tear production value obtained using the PRT was 23.59 ± 5.90 mm, and the average OSDI score was 7.11 ± 6.20.
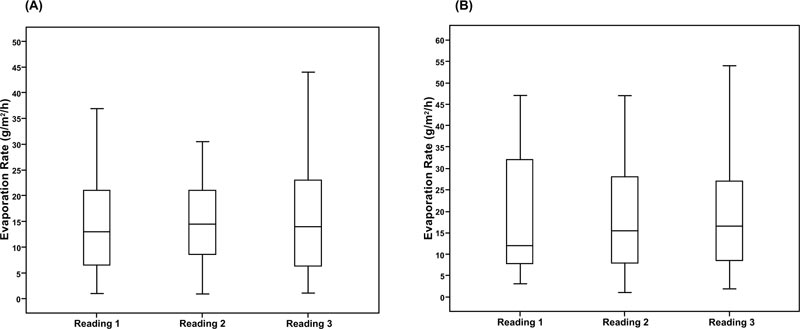
The three tear evaporation rate measurements obtained by E1 at visit 1 are shown in Fig. (1A). The mean of these three readings was 19.38 ± 0.79 g/m2/h while the mean of the three tear evaporation readings obtained by E2 at visit 2 was 20.49 ± 0.48 g/m2/h (Fig. 1B). The mean values for the three evaporation rate readings obtained at each visit are shown in Table 1.
Using the VapoMeter, no significant difference was noted between the three readings measured at visit 1 (p = 0.68, Friedman test). Similarly, no significant difference was observed between the three readings obtained at visit 2 (p = 0.73, Friedman test).
The average ICC over the three readings of evaporation rate was 0.84 and 0.63 with a 95% confidence interval (CI) at visits 1 and 2, respectively. A comparison of the reliability of the measurements from the two examiners (reproducibility) showed an ICC of 0.69 with a 95% CI. No statistically significant correlation was found between the tear parameters assessed in this study (Fig. 2).
4. DISCUSSION
This study assessed the repeatability and reproducibility of the tear film evaporation rate measurement using a commercially available device. The development and availability of a new technology, the VapoMeter (Delfin Technologies, Finland) can help clinicians evaluate tear film evaporation easily and rapidly. Tear evaporation rate readings are produced within seconds, without contact, or disturbance of, tear film layers.
Since 1980, many studies have been conducted using various methods to measure the tear evaporation rate [19]. Methods including measuring fluid loss from the ocular surface and interferometric measurements of tear film thinning have been widely used to estimate the tear evaporation rate [20, 21]. Due to the use of different techniques, the evaporation rate is reported in different units, including µL/min, g/m2/h, and 10-7 g/cm2/s [19].
Numerous attempts have been made to measure the tear evaporation rate in healthy individuals and those with dry eyes. Studies have shown that the evaporation rate in healthy individuals ranged between 0.04 and 0.07 µL/min (14.4 and 25.2 g/m2/h) [20, 22, 23]. However, studies conducted in the 1990s have estimated a higher evaporation rate among healthy individuals compared to more recent studies. These studies have indicated that values between 0.11 and 0.16 µL/min (39.6 and 57.6 g/m2/h) are considered the normal range of the evaporation rate [10, 24].
However, the conflict between studies regarding the tear evaporation rate could be due to the fact that tear evaporation measurement is strongly influenced by the surrounding environment. Many studies have shown that the evaporation rate of the tear film was affected by the changes in relative humidity [12, 25]. Furthermore, other factors, such as ambient temperature and airflow, affect the measurement of the tear evaporation rate [26]. Therefore, the conflicting results between studies could result from the differences in environmental factors, such as humidity, temperature, and airflow. In this study, humidity and temperature were monitored during evaporation rate measurements to minimize variations induced by the ambient environment.
| Visit 1 | Visit 2 | |
| Reading 1 | 18.49±18.26 | 20.49±17.75 |
| Reading 2 | 19.46±18.9 | 20.95±19.84 |
| Reading 3 | 20.00±20.77 | 19.79±14.71 |
Measurement of the evaporation rate is an essential tool in the differential diagnosis of dry eye syndrome [19]. Several studies have shown that tear evaporation is greater in patients with aqueous deficiency and meibomian gland dysfunction. Furthermore, studies have suggested that water loss from the ocular surface ranges between 0.1 and 0.24 µL/min (36 and 86 g/m2/h) in patients with dry eyes [27, 28].
This study found that the mean tear evaporation rate in healthy participants measured using the VapoMeter was 19.38 and 20.49 g/m2/h (0.05 and 0.06 µL/min) at visits 1 and 2, respectively. These results conform to the findings of other studies that have found that the tear evaporation rate in healthy participants was between 0.04 and 0.07 µL/min [19, 23, 29]. This also conforms to earlier observations, which have shown that the mean tear evaporation rate in healthy participants was 20.97 g/ m2/h [22].
This study showed that performing a tear evaporation test using the modified evaporimeter in healthy participants produced consistent tear evaporation measurements without significant differences between the measurements obtained in different sessions by the same examiner and those obtained by other examiners (p > 0.05). The ICC reliability test indicated a moderate to good repeatability (ICC = 0.89 with a 95% CI) and reproducibility (ICC = 0.69 with a 95% CI) of tear film evaporation rate measurements using the VapoMeter.
CONCLUSION
The results of this study suggest that the VapoMeter provides repeatable and reproducible measurements of the tear film evaporation rate. This study showed that the VapoMeter could provide clinicians with a readily available method for rapid evaluation of the tear film evaporation rate. Measuring tear evaporation rate using VapoMeter eliminates many of the previously reported disadvantages of old techniques, such as prolonged testing time and the complicated setup and modification processes needed. The handheld VapoMeter can be used to measure the tear film evaporation rate for the possible diagnosis of evaporative dry eye. By considering the significance of tear evaporation as a diagnostic tool for dry eye syndrome, this instrument may help clinicians to diagnose better and manage dry eye syndrome.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the College of Applied Medical Science Research Center, King Saud University, Saudi Arabia under ethical approval no. CAMS 002a-3839.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants after explaining the study procedure.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The authors extend their appreciation to the Cornea Research Chair, Optometry Department, College of Applied Medical Sciences, King Saud University for its funding for this research.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors extend their appreciation to the Cornea Research Chair, Optometry Department, College of Applied Medical Sciences, King Saud University for its funding for this research.


