All published articles of this journal are available on ScienceDirect.
Comparison of Visual Outcomes and Patient Satisfaction Following Cataract Surgery with Two Monofocal Intraocular Lenses: Clareon® vs AcrySof® IQ Monofocal
Abstract
Aim:
This study aimed to compare the performance of two monofocal Intraocular Lenses (IOL) platforms.
Background:
The Clareon® Intraocular Lens (IOL) is a relatively new monofocal lens platform designed to improve postoperative results compared to other monofocal platforms.
Objective:
This study aimed to assess and compare the visual and refractive outcomes, and incidence of YAG capsulotomy of the Clareon® IOL and a standard non-preloaded AcrySof® monofocal IOL following contralateral implantation in patients undergoing cataract surgery.
Methods:
A total of 20 patients (40 eyes; 12 female, average age 72.8±6.4 years) who had undergone contralateral implantation of an AcrySof® IQ monofocal lens (SN60WF or SN6AT; Alcon; Texas, USA) and a Clareon®monofocal lens (CNAOT0; Alcon; Texas, USA) were selected. Uncorrected Distance Visual Acuity (UDVA), Contrast Sensitivity (CS), kinetic perimetry, and refraction were measured 1 month following the second surgery and subjective vision was measured 6 months following the second surgery using a quality-of-life questionnaire.
Results:
There was no difference in postoperative UDVA (P=0.94), CS (P>0.05), or refraction (P=0.64) between eyes that received the Clareon® and AcrySof® IQ lenses. Clareon® eyes had a higher incidence of glare/haloes and positive dysphotopsia while AcrySof® IQ eyes had a higher incidence of negative dysphotopsia. Patient satisfaction was similar between the groups (P=0.86), although 25% of patients reported more clarity in the eye that received the Clareon® lens. The incidence of posterior capsular opacification was low for both groups.
Conclusion:
Clareon® and AcrySof® IQ lenses perform similarly, providing good refractive, visual, and subjective outcomes. Clareon® is available as a preloaded lens option and may reduce PCO and the need for Nd: YAG capsulotomy.
1. INTRODUCTION
Monofocal lenses are the most common type of Intraocular Lens (IOL) implanted during cataract surgery due to their predictable outcomes and low risk of visual artifacts. The Clareon® IOL is a relatively new aspheric hydrophobic acrylic monofocal IOL that claims to improve visual outcomes compared to other monofocal IOLs.
Glare and positive dysphotopsias are caused largely by light reflecting off the edge or interior surfaces of the IOL onto the retina. They are usually associated with square edge IOL designs but can also be caused by peripheral non-imaging optic geometry [1]. Negative dysphotopsia is less understood but is believed to be caused by a gap between the retinal images formed by light bypassing the IOL and light passing through the IOL optic [2]. While positive dysphotopsia tends to not improve with time, negative dysphotopsia typically resolves on its own for most patients. It is difficult to predict who will develop photic symptoms following cataract surgery, although patients who are predisposed to haloes, e.g. patients with high preoperative Higher-Order Aberrations (HOAs), tend to be more susceptible to positive dysphotopsia while patients with a large functional retina may be more susceptible to negative dysphotopsia [3]. Small pupils and large angle kappa and angle alpha may also increase the likelihood of dysphotopsia [2, 3]. IOL designs have been adapted over time to prevent these symptoms. While square edge designs are successful at preventing Posterior Capsular Opacification (PCO), they cause the bending and reflection of light that results in photic phenomena. In response, more recent IOL designs have incorporated frosted or curved anterior edges. Larger optics, lower refractive power [3], positioning of the haptic junctions at 3 o’clock and 9 o’clock positions [4], and reverse optic capture technology [5], where the IOL overlays the capsulotomy edge, all help to prevent dysphotopsia. Visual outcomes can also be affected by glistenings, small vacuoles that form in the IOL over a period of years following surgery. Glistenings cause stray light and scattering, thereby degrading image quality and low light visual acuity [6].
The first AcrySof® platform became available in 1990 in the UK and since then, millions of AcrySof® IOLs have been implanted globally. While results are generally predictable with the AcrySof® IOLs, some criticisms on the platform include issues with glistenings, surface haze, and dysphotopsia, as well as risks associated with a platform that is not preloaded, such as intraoperative scratches to the optic. To overcome these issues, the Clareon® platform and AutonoMe™ loading device were released in 2018.
The Clareon®IOL is an asymmetric, biconvex lens composed of hydrophobic acrylic material. It may prevent photic phenomena by way of its precision square edge design and fully functional 6 mm optic. The square edge may also prevent PCO by inhibiting epithelial cell migration. The lens is made of a novel hydrophobic acrylate/methacrylate copolymer that may improve clarity, and reduce surface haze, roughness and glistenings compared to other monofocal IOLs. The lens comes preloaded in the AutonoMe™ device, an innovative disposable delivery system powered by a carbon dioxide activated pump mechanism, which has been reported to promote less corneal inflammation and less endothelial cell loss compared to other preloaded cartridges [7].
Due to its relatively recent commercial availability, limited data is available regarding how the Clareon® lens behaves in situ; the bulk of available data comes from in vitro studies. This paper compares the visual outcomes of Clareon® and AcySof® IQ IOLs implanted contralaterally in patients undergoing cataract surgery, assessing visual acuity, photic phenomena, the incidence of PCO, and patient satisfaction in a group of 20 patients over 12 months.
2. METHODS
A total of 20 patients (40 eyes) were recruited for the study (12 females, average age 72.8±6.4 years). Patients were considered for inclusion if they had preoperative cylinder less than 1 D, had undergone bilateral routine phacoemulsification, and contralateral implantation of an AcrySof® monofocal IOL (SN60WF or SN6AT; Alcon; Texas, USA) and a Clareon® monofocal IOL (CNAOT0; Alcon; Texas, USA). Patients were excluded for consideration if they had any underlying ocular pathology that may affect visual function (e.g. macular degeneration, glaucoma, epiretinal membrane, amblyopia, diabetic eye disease), experienced intra- or postoperative complications, preoperative cylinder exceeding 1 D, or received monovision or had vision corrected for near.
All surgeries were performed by a Single Surgeon (SA), whereby routine phacoemulsification and implantation of an AcrySof® monofocal IOL (SN60WF or SN6AT; Alcon; Texas, USA) and a Clareon®monofocal IOL (CNAOT0; Alcon; Texas, USA) were performed on contralateral eyes for each patient. Biometry measurements were performed using an IOLMaster 700 (Zeiss; Germany), and lens power was calculated using Barrett or Barrett toric (for eyes receiving a toricAcrySof® IOL) formula using target refraction of plano±0.25 D for each eye. The procedures were performed under local or general anesthesia as per patient selection. Two 1 mm paracenteses were made at 180º and a 2.3 mm and the main incision was made temporally. A 26-gauge cystotome was used to create a continuous curvilinear capsulorhexis. Following the surgery, patients were advised to commence ofloxacin (Allergan; Ocuflox; Dublin, Ireland) and prednisolone acetate/phenylephrine hydrochloride (Allergan; Prednefrin forte; Dublin, Ireland) eye drops following the surgery, applying one drop 2-hourly until bed-time. From the following day, the patients were advised to continue using ofloxacin and prednisolone acetate/phenylephrine hydrochloride eye drops applying one drop qid for 2 weeks and 3-4 weeks, respectively, and to commence using ketorolac (Allergan; Acular; Dublin, Ireland) eye drops applying one drop qid for 3 days. Patients attended a 1-month postoperative appointment within 4-6 weeks following the second surgery, where Uncorrected Distance Visual Acuity (UDVA), uncorrected Contrast Sensitivity (CS) (contrast sensitivity function i.e. CSF and modulation transfer function i.e. MTF), and refraction were measured. Visual acuity was measured under photopic conditions using a Snellen chart placed at 6 metres. Contrast sensitivity was measured using a sinus grating chart under mesopic conditions for CSF, and MTF was measured using an autorefractor (iTrace; Tracey Technologies LLC; Texas, USA). Kinetic perimetry was used to objectively measure the incidence of negative dysphotopsia [8] and was performed on a Humphrey Field Analyser 3 (Zeiss; Germany) using a white III 4 e stimulus with the variable speed at the following meridians: 0°, 30°, 90°, 150°, 180°, 240°, 270°, and 300°. The subjective vision was measured 6 months following the final surgery using a quality of vision questionnaire adapted from the PseudophakicDysphotopsia Questionnaire previously published by [9]. Subjective patient satisfaction was recorded as a number out of 10, where 10 denotes very satisfied and 0 denotes very dissatisfied. Mean values recorded at the final 4-6 week postoperative appointment (i.e. following the second surgery) were compared between the Clareon® and AcrySof® IQ monofocal groups using paired T-tests and the Excel data analysis tool (Microsoft Corporation; Washington, USA) where P<0.05 was assumed statistically significant. Lens specifications are listed in Table 1.
| - | Clareon® | AcrySof® IQ |
|---|---|---|
| Optic type | Asymmetric biconvex | Aspheric, biconvex |
| Optic material | Hydrophobic acrylic | Hydrophobic acrylic |
| Optic diameter | 6 mm | 6 mm |
| Overall length | 13 mm | 13 mm |
| Haptic angulation | 0° planar | 0° |
| Haptic configuration | STABLEFORCE™ | STABLEFORCE™ |
| Photoprotection | UV and blue light filtration | UV and blue light filtration |
| Refractive index | 1.55 | 1.55 |
| Edge curvature | 7.9 µm | 8.5 µm (SN60WF); 9.3 µm (SN6AT) |
| Water content | 1.5% | 0.5% |
| A Constant | 119.1 | 119.0 (SN60WF); 119.2 (SN6AT) |
| - | Clareon® (n=20 eyes) | AcrySof® IQ monofocal (n=20 eyes) | P value |
|---|---|---|---|
| Age | 72.8±6.4 | - | |
|
Gender (female male) |
12 8 |
- | |
| CDVA (LogMAR) | 0.19±0.16 | 0.25±0.14 | 0.26 |
| SE | 1.26±1.5 | 1.27±0.98 | 0.98 |
| Sphere | 1.7±1.4 | 1.6±1.1 | 0.85 |
| Cylinder | -0.6±0.7 | -0.6±0.5 | 0.71 |
| - |
Clareon (n=20 eyes) |
AcrySof® IQ monofocal (n=20 eyes) |
P value |
|---|---|---|---|
| UDVA (LogMAR) | 0.03±0.06 | 0.03±0.07 | 0.94 |
| CDVA (LogMAR) | 0.01±0.02 | 0.02±0.05 | 0.40 |
| SE | 0.03±0.4 | -0.04±0.3 | 0.64 |
| Sphere | 0.12±0.4 | 0.08±0.5 | 0.82 |
| Cylinder | -0.46±0.4 | -0.31±0.2 | 0.29 |
3. RESULTS
Each group had similar preoperative Corrected Distance Visual Acuity (CDVA) (P=0.26) and refraction (P=0.98) (Table 2). Of the 20 eyes that had received AcrySof® IQ lenses, three eyes were implanted with SN6AT2, three eyes with SN6AT3, and one eye with SN6AT4, while all remaining eyes (13 eyes) were implanted with non-toric SN60WF lenses. No patients experienced peri- or postoperative complications, and no lenses were needed to be explanted.
3.1. Refraction and Visual Acuity
There was no difference in average postoperative refraction between the groups (P=0.64), with 15 eyes (83%) and 12 (86%) eyes that received Clareon® lenses and AcrySof® IQ lenses, respectively, achieving postoperative spherical equivalent (SE) within ±0.5 D of the target refraction (Fig. 1A). There was no difference in postoperative monocular UDVA (P=0.94) or CDVA (P=0.4) between the two groups, with all patients achieving postoperative UDVA of 6/9 (equivalent to 20/30 or 0.2 LogMAR) or better in each eye (Fig. 1B).
3.2. Contrast Sensitivity
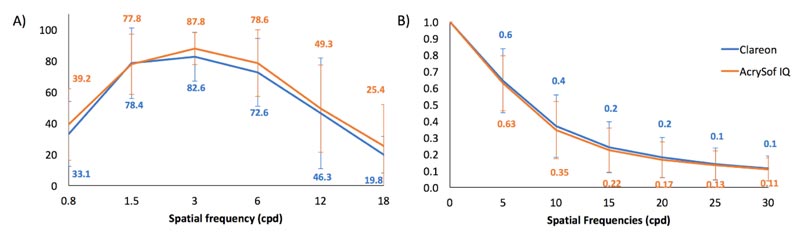
There was no difference in CSF (Fig. 2A) or MTF (Fig. 2B) between the two groups (P>0.05).
3.3. Photic Phenomena and Subjective Vision
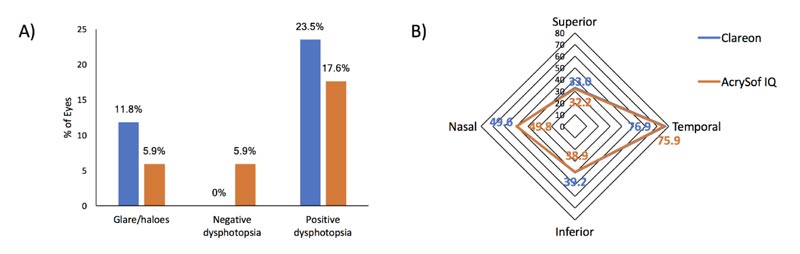
Average satisfaction was similar for both groups; 8.4 for Clareon® eyes and 8.8 for AcrySof® IQ eyes (P=0.86). However, 5 patients (25%) reported superior clarity in the eye that received the Clareon® lens, while 1 patient (5%) reported superior clarity in the eye that received the AcrySo® IQ lens and 70% reported similar clarity in both eyes. Higher numbers of patients who received Clareon® lenses experienced glare/haloes and positive dysphotopsia, however, no patients from this group reported negative dysphotopsia (Fig. 3A). No restriction in the visual field was detected using kinetic perimetry for either group (Fig. 3B).
3.4. PCO
No patients were presented with PCO in the eye that received the Clareon® IOL within the3 years following surgery. Four patients (20%) were presented with posterior capsular fibrotic changes in the eye that received the AcrySof® IQ monofocal IOLs with 3 patients (15%) requiring Nd: YAG capsulotomy within three years following surgery.
4. DISCUSSION
Monofocal IOLs are the most commonly implanted type of IOL due to their predictable postoperative results and low incidence of photic phenomena compared to multifocal IOLs. Clareon® is a relatively new preloaded monofocal IOL platform that may help to enhance clarity and with reduced incidence of PCO and dysphotopsia. However, little is known regarding how the lens behaves in situ as previous studies have relied largely on in vitro experimental techniques due to the relatively recent commercial availability of the lens. To our knowledge, this is the first study to directly compare the performance of Clareon® and AcrySof® IQ IOLs in situ following contralateral implantation in a group of 20 human subjects undergoing cataract surgery.
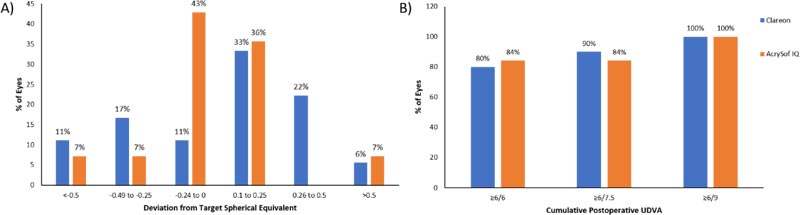
This study assessed patients who had undergone contralateral implantation of an AcrySof®IQ IOL and a Clareon® IOL, and who fulfilled the inclusion criteria. Both groups achieved similar average postoperative spherical refraction and UDVA (Table 3), and a similar proportion of eyes within ±0.5 D of the target (Fig. 1A). Visual acuity and refractive results largely followed a normal distribution. Two outliers (considered to be > ±0.5 D from target SE) were present in each group for postoperative SE, caused by residual astigmatism. Recruitment was restricted to patients with preoperative cylinder less than 1.0 D to prevent residual astigmatism, as toric versions of the Clareon®were not available at the time of the study. However, as toric lenses were selected for the first eye of each patient if the preoperative cylinder was >0.75 D, 7 of the 20 eyes that received AcrySof® IQ lenses received toric SN6AT lenses. This may have resulted in the residual postoperative cylinder in eyes that received Clareon® lenses, although there was no statistical difference between the groups postoperatively (P = 0.29) and outliers were present in both groups. Both groups were also within the guidelines recommended by the Royal College of Ophthalmologists; 85% within ±1.0 D and 55% within ±0.5 D of the target refraction [10]. Long-term refractive stability was stable up to 12 months. However, in vitro analysis has reported less axial displacement and related dioptric power shift for SN60WF and Clareon® IOLs compared to other IOLs [11], suggesting that refraction is likely to remain stable long-term following implantation.
Quality and clarity of vision are dependent on IOL material, surface qualities, and refractive index. While AcrySof® IQ and Clareon® IOLs are both composed of a similar hydrophobic acrylate/methacrylate copolymer material with a refractive index of 1.55, Clareon® IOLs have a slightly higher water percentage (i.e. 1.5% versus 0.5%). Analysis of artificially aged Clareon® lenses have shown improved surface smoothness and decreased glistenings compared to competitor lenses [12]. This is likely due to the high-water content of the lens, resulting in less light scatter, improving image quality, and maintaining CS. A higher proportion of patients (i.e. 25% vs 5%) reported superior clarity in the eye that received Clareon® IOL, which is consistent with previous reports and likely due to superior surface smoothness. There was no difference in CSF or MTF between the two groups for this study (Figs. 2A, B). As glistening develop over time, increasing for up to 10-15 years following implantation [6], it is not unexpected to see no difference within the first 3 years following surgery. To fully assess postoperative CS, and incidence of glistenings and PCO, a long-term study will be required.
Both AcrySof® IQ and Clareon® lenses feature square edge designs that aim to prevent PCO by inhibiting epithelial cell migration. Studies that have compared the incidence of PCO following implantation of SN60WF and Clareon® IOLs in rabbit and explanted capsular bags from human cadavers reported no difference in PCO incidence or progression rates [13, 14]. However, a large-scale meta-analysis reported that incidence of PCO requiring Nd: YAG capsulotomy within the first year of surgery in humans was higher in eyes that received SN60WF lenses compared to Clareon® lenses (1.44% versus 0.62%, respectively) [15]. This study reported Nd: YAG capsulotomy incidence rate of 15% in eyes that received AcrySof® IQ lenses, while no PCO was recorded in eyes that received Clareon® lenses within 3 years of the final surgery. The first patient to develop PCO had a posterior subcapsular cataract preoperatively, so the opacification is likely to have been caused by posterior capsular fibrosis. Since then, no further patients have presented with complaints of PCO symptoms. A low incidence of PCO was expected in Clareon® eyes due to the precise square edge design of the IOL. Incidence in eyes that received the AcrySof® IQ IOLs was higher than previously reported [16]. The small sample size of this study and the inclusion of patients with posterior subcapsular cataracts preoperatively make these results difficult to interpret reliably.
Photic phenomena, such as glare/haloes and dysphotopsias, are in part contributed to straight edge optic designs and peripheral non-imaging optic geometry [1]. Photic phenomena were measured using two methods; subjective questionnaire and kinetic perimetry. For subjective symptoms, a questionnaire was used that was adapted from the Pseudophakic Dysphotopsia Questionnaire previously published by Kinard et al. [9]. While negative dysphotopsia was only reported in eyes that received AcrySof® IQ lenses, positive dysphotopsia was more commonly reported in eyes that received Clareon® IOLs compared to eyes that received AcrySof® IQ IOLs (23.5% versus 17.6%, respectively; Fig. 3A). Lens edge designs are a trade-off; a sharp edge prevents PCO but induces positive dysphotopsia. To limit positive dysphotopsia, IOL designs have introduced frosted or curved edges to prevent the reflection of light back onto the IOL. Clareon® lenses have a lower radius of curvature on the posterior edge (7.9 µm versus 8.5 µm and 9.3 µm for SN60WF and SN6AT, respectively) [17]. Although slight, this difference could explain the higher incidence of positive dysphotopsia as well as the lower rate of capsular fibrosis in eyes that received Clareon® lenses. The etiology of negative dysphotopsia is less understood but is postulated to be caused by the bending of incoming light resulting in a gap forming between the retinal images produced by light bypassing the lens and light being refracted by the optic [2]. Methods to objectively measure dysphotopsia have traditionally been elusive. However, kinetic perimetry has been used to objectively measure negative dysphotopsia symptoms [8]. Briefly, Goldmann kinetic perimetry was used to measure the visual field of pseudophakic patients, and temporal field restrictions and temporal scotomas were detected that corresponded with the location of subjectively reported shadows [8]. When measured subjectively, 5.9% of patients reported experiencing negative dysphotopsia in the eye that received AcrySof® IQ lens (Fig. 3A). No patients reported such symptoms in the eye that received the Clareon® lens (Fig. 3A). However, when measured using kinetic perimetry, there was no difference in visual fields between the groups, with both groups lying within the population norm (Fig. 3B). This may suggest discrepancies between visual field defects and symptoms perceived by the patient or may suggest that temporal field restriction as measured by kinetic perimetry is not sensitive enough to reliably predict dysphotopsia symptoms in a sample group of this size. This may be caused by the fact that standard kinetic perimetry tests only measure up to 90° of the peripheral visual field. Despite differences in photic symptoms, both groups reported similar rates of satisfaction (8.4 and 8.8 out of 10 for Clareon® and AcrySof® IQ eyes, respectively; P>0.05), suggesting that such symptoms may not be clinically significant or impact quality of life.
The Clareon® IOL comes preloaded in the AutonoMe™ delivery system. The automated preloaded delivery system of the AutonoMe™ device has been reported to inflict less wound trauma during IOL insertion compared to manual insertion, leading to reduced incidence of Descemet membrane detachment, posterior gape, and wound retraction [18]. According to the surgeon, during this study, the delivery system appears to be precise and predictable with few minor complications. The carbon dioxide pump failed in one case and the trailing haptic was found to be stuck to the optic in a few cases. The haptic could be easily removed from the optic and no further intra- or postoperative complications were encountered for any patient. All IOLs appeared well centered in the eye following surgery and remained stable at follow-up.
Limitations of this study include a small sample size due to a limited patient pool as patients were recruited from the cohort that had incidentally undergone contralateral implantation of an AcrySof® IOL and a Clareon® IOL as a result of a change in surgeon preference and protocol between the surgeries, and relatively short follow-up time. Some complications, such as glistenings, which can reduce contrast sensitivity, can take 5-10 years to develop while clinically significant PCO may take 4-5 years. Therefore, a long-term comparative study will be required to effectively compare CS and the incidence of PCO. However, no patients have presented until recently complaining of PCO symptoms in the eye that received the Clareon® IOL. Additionally, the inclusion of eyes with toric IOLs may have skewed photic phenomena resulted as toric IOLs are implanted along the steep axis while monofocal IOLs are implanted inferotemporally to minimise photic phenomena as previously reported and as per surgeon preference [4]. Toric versions of the Clareon® IOL were not available at the time of the study, but are now readily available. Allowing treatment of astigmatism in the eyes that received Clareon® IOLs may have improved postoperative visual outcomes, although there was no statistically significant difference in postoperative refraction between the groups (P = 0.64).
CONCLUSION
The Clareon® IOL does not appear to be inferior to the AcrySof® IQ IOL, achieving similar visual, refractive, and subjective outcomes. However, the lower radius of curvature on the posterior edge of the Clareon® lens, while potentially leading to lower incidence of PCO and Nd; YAG capsulotomy, may induce more positive dysphotopsia than AcrySof® IQ lenses. Benefits of the Clareon® system over other monofocal IOLs may include improved visual clarity, lower incidence of Nd: YAG capsulotomy for PCO, and ease-of-use of the AutonoMe™ delivery system. Due to the small size of this study, larger long-term prospective studies will be required to help elucidate, as well as to determine differences in the long-term incidence of glistenings and PCO between the lens platforms.
LIST OF ABBREVIATIONS
| IOL | = Intraocular Lens |
| UDVA | = Uncorrected Distance Visual Acuity |
| CS | = Contrast Sensitivity |
| HOAs | = Higher-Order Aberrations |
| PCO | = Posterior Capsular Opacification |
| CSF | = Contrast Sensitivity Function |
| MTF | = Modulation Transfer Function |
| CDVA | = Corrected Distance Visual Acuity |
| SE | = Spherical Equivalent |
ETHICAL STATEMENT
Ethics approval was not required according to the applicable HREC committee due to the retrospective nature of the study. This research was carried out according to the tenets of the declaration of helsinki.
CONSENT FOR PUBLICATION
Informed written consent to participate and consent for publication were obtained from all participants.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
No financial support was received for this study. Dr. Erin thornell has no conflicts of interest to disclose. Dr. Smita agarwal is a member of the advisory board for alcon, the manufacturer of both of the lenses used for this study.
ACKNOWLEDGEMENTS
Declared none.


