RESEARCH ARTICLE
Assessment of the Efficiency of HP-Guar and hyaluronic Acid Tear Supplements to Control Tear Film Evaporation Rate in Dry Eye Subjects
Ali Abusharha1, *, Abdulrhman A. Shbear2, Raied Fagehi1, Mana A. Alanazi1, Ali Alsaqr1, Gamal A. El-Hiti3, Ali M. Masmali3
Article Information
Identifiers and Pagination:
Year: 2021Volume: 15
First Page: 299
Last Page: 304
Publisher ID: TOOPHTJ-15-299
DOI: 10.2174/1874364102115010299
Article History:
Received Date: 30/03/2021Revision Received Date: 11/10/2021
Acceptance Date: 03/11/2021
Electronic publication date: 28/12/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
The most common factor that could lead to dryness is the accelerated tear evaporation rate. Controlling the tear evaporation rate is increasingly used as a method to control dry eye complications. The present study explores the effects of different tear supplements formulations on tear film evaporation rate.
Objective:
This study aimed to evaluate the short-term effects of Systane ULTRA and Artelac Advanced eye drops on the tear film evaporation rate.
Methods:
Fifteen male dry eye subjects were enrolled in the current study. Tear film parameters were observed at several time points post installation (10, 20, 30, and 60 min). The tear film parameters observed in the current study were tear evaporation rate, noninvasive breakup time (NITBUT) and tear meniscus height (TMH). Two visits were required to conduct this study. One visit was conducted to assess the physiological tear film parameters with the use of Systane® ULTRA eye drop. The other visit was conducted to assess tear film parameters with the use of Artelac Advanced eye drop.
Results:
The mean tear evaporation rate at baseline was 52.58 ± 23.24 g/m2 h. A box plot of tear evaporation showed a reduction in tear film evaporation rate after instillation of Systane eye drop. A drop in tear film evaporation rate of 14% was observed at 20 and 60 min time point after instillation of Systane ULTRA eye drop. A significant increase in NITBUT was found after instillation of Systane ULTRA (P = 0.01) and Artelac Advanced (P = 0.02).
Conclusion:
The current study indicates a significant improvement in the tear film parameters using both HP-Guar and hyaluronic acid formulations. However, it was apparent that the use of HP-Guar was superior to hyaluronic acid in controlling the tear evaporation rate in dry eye subjects.
1. INTRODUCTION
A healthy, stable preocular tear film plays a vital role in protecting, moisturizing, and nourishing the cornea. Moreover, it helps maintain clear vision as it is considered the primary refracting surface in the visual system [1]. Tear film abnormalities could be caused by either internal or external factors. Tear hyperosmolarity and tear film instability are believed to be the core mechanisms of the cycle of the events leading to dry eye complications [2].
Attempts have been made to control and manage complications of dry eye syndrome. Methods such as artificial tears, lacrimal punctal occlusion, and anti-inflammatory therapy have been widely used for the management of dry eye [3, 4]. Artificial tears are one of the most commonly used therapies that work by replacing tears and lubricating the ocular surface. Additionally, it helps in restoring corneal surface and goblet cell density and improving visual acuity [4].
However, the main drawback of tear supplements is the short retention time in the eye due to the dynamic mechanism of tear film drainage providing temporary relief of dry eye complications [5]. Therefore, several attempts have been made to increase the bioavailability of tear film supplements in the ocular surface and prolong the contact time between the tear supplement and the ocular surface [6]. Therefore, preparations containing mucoadhesive polymers have been used to prolong the residence of the ophthalmic solution in the eye [7]. The duration of residence of bioadhesive polymers depends mainly on their classification, whether water-soluble or insoluble. The residence time of water-soluble polymers depends on the dissolution rate of the polymer, while that of water-insoluble polymers depends on the mucus turnover rate [8].
Hydroxypropyl-Guar (HP-Guar) gellable lubricant eye drops are a gelling polymer formulation that has recently been used to enhance solution retention. Systane is a commercial tear supplement containing HP-Guar. It has been designed to act as mucomimetic utilizing the concept of pH-sensitive HP-Guar formulation (350) [9]. Systane has a pH of 7 in the bottle, where the average pH of the tears is 7.50 [10, 11]. Once Systane is installed into the eye, HP-Guar changes from low-viscosity liquid to gel due to the difference in pH between the drop bottle and the eye [11].
Other mucomimetic formulations that contain hyaluronic acid have been proposed to increase the retention time of the eye drop in the eye. Hyaluronic acid is a common component of synovial fluids and extracellular matrix [12, 13]. In the human body, the highest content of hyaluronic acid is found in the umbilical cord and vitreous humour of the eye [13, 14]. Moreover, hyaluronic acid can be found in the human skin, joints, and other connective and epithelial tissues [14].
Hyaluronic acid obtained its viscosity properties from its high molecular weight that may reach millions of Daltons [15]. This explains the high moisture retention capacity of hyaluronic acid [13]. Hyaluronic acid has been widely used to manage dry eye syndrome and protect the ocular surface [16]. Studies have shown that sodium hyaluronic acid improved dry eye symptoms by improving tear film stability and moisturizing the ocular surface [17]. Moreover, hyaluronic acid plays an important role in ocular tissue healing following ophthalmic surgery, such as cataract and glaucoma surgeries [18].
This study aimed to evaluate the short-term effects of different tear supplement formulas on the tear film parameters namely, tear evaporation rate (TER), noninvasive breakup time (NITBUT), and tear meniscus height (TMH). In this study, the efficacy of ophthalmic formulations containing HP-Guar gellable lubricant eye drops and hyaluronic acid was assessed.
2. MATERIALS AND METHODS
The effect of two different lubricant eye drops on tear film parameters was assessed in this study. All procedures were approved by the College of Applied Medical Sciences Ethics Committee, King Saud University, Riyadh, Saudi Arabia. All subjects provided written informed consent. The study was conducted according to the tenets of the Declaration of Helsinki.
This non-randomized, observational, and comparative study included fifteen male dry eye subjects (mean ± standard deviation (SD) = 35.7 ± 3.27 years). Subjects with an Ocular surface disease index (OSDI) score > 10 and a tear film breakup time < 10 s were denied entry. To monitor the changes in tear film behavior after the instillation of tear supplements, tear film parameters were observed at several time points post-instillation (10, 20, 30, and 60 min). The measurements were carried out by the same examiner in a controlled environment in terms of temperature and humidity. Subjects with signs of ocular infection, abnormalities, and those recently used ocular and systemic medications were excluded.
Two visits separated by one-week wash-out periods were required to conduct this study. One visit was conducted to assess the physiological tear film parameters with the use of Systane ULTRA eye drop (Alcon Laboratories, Fort Worth, Texas, USA). The other visit was conducted to assess tear film parameters with the use of Artelac Advanced eye drop.
The TER was measured using VapoMeter (Delfin Technologies, Kuopio, Finland) [19]. Two readings were obtained, first with eyes open, which represent the evaporation rate from the ocular surface and lid skin. Then, a second measurement was obtained with eyes closed, and these measurements were subtracted from the open-eye readings. Three measurements were obtained; then, the average value was calculated. To monitor the change in the NITBUT and TMH post-instillation, the OCULUS Keratograph 4 (OCULUS Inc., Wetzlar, Germany) was used [20].
All data were statically analyzed using SPSS Statistics version 23 (IBM corporation, Somers, NY, USA). A test of normality was carried out first using a Kolmogorov-Smirnov test. Normally distributed data were compared using a repeated measured ANOVA and Tukey’s post-hoc test. Data not normally distributed were compared using Friedman’s test and a post-hoc Wilcoxon rank sum test.
3. RESULTS
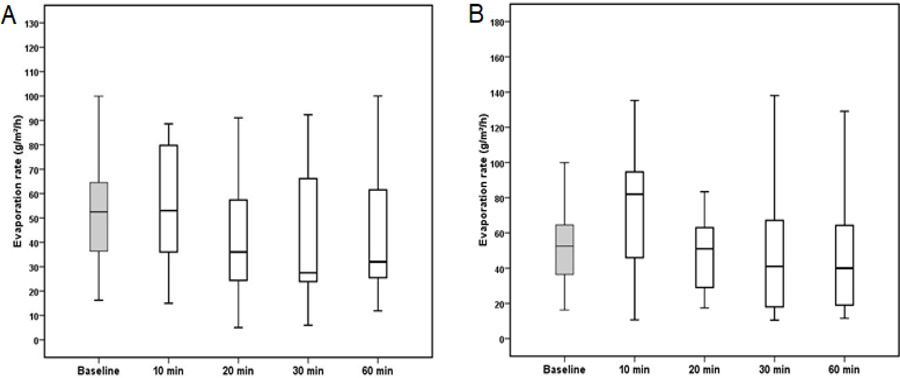
The tear film evaporation rate measurements after the instillation of Systane ULTRA (P = 0.059) and Artelac Advanced (P = 0.060) were normally distributed. A box plot of TER showed a reduction in tear film evaporation rate after the instillation of Systane eye drop (Fig. 1). A decrease in tear film evaporation rate of 14% was observed after 20 and 60 min from the instillation of Systane ULTRA eye drop. The mean and standard deviation of the TER measured at different time points are shown in Table 1.
| Time (Minute) | Baseline | 10 | 20 | 30 | 60 |
|---|---|---|---|---|---|
| Systane ULTRA | 52.58 ± 23.24 | 55.02 ± 26.73 | 45.41 ± 30.56 | 47.31 ± 34.19 | 43.27 ± 26.05 |
| Artelac Advanced | 52.58 ± 23.24 | 73.51 ± 36.15 | 55.73 ± 40.88 | 52.38 ± 44.50 | 56.90 ±55.09 |
| Time (Minute) | Baseline | 10 | 20 | 30 | 60 |
|---|---|---|---|---|---|
| Systane ULTRA | 5.36±1.21 | 6.91 ± 2.86 | 6.39 ± 2.62 | 7.58 ±4.47 | 6.87 ± 4.20 |
| Artelac Advanced | 5.36±1.21 | 9.36 ± 5.45 | 6.99 ± 3.40 | 8.37 ±4.63 | 6.65 ± 3.63 |
| Time (Minute) | Baseline | 10 | 20 | 30 | 60 |
|---|---|---|---|---|---|
| Systane ULTRA | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.02 | 0.16 ± 0.02 |
| Artelac Advanced | 0.15 ± 0.01 | 0.16 ± 0.03 | 0.17 ± 0.02 | 0.16 ± 0.02 | 0.15 ± 0.02 |
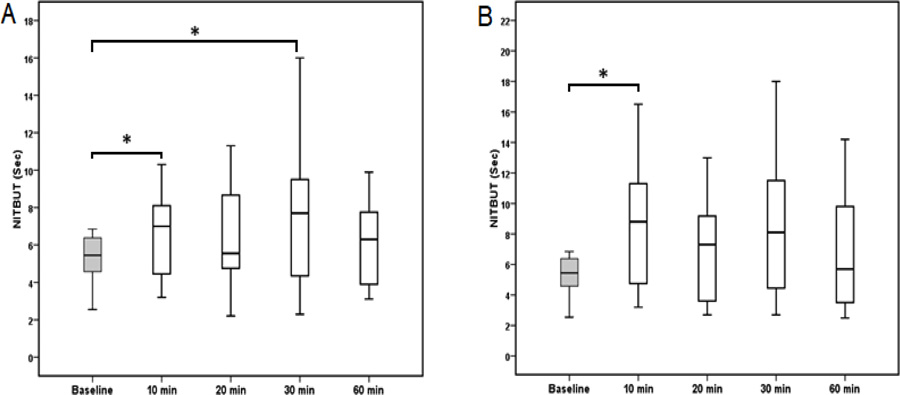
A box plot of NITBUT is shown in (Fig. 2). Unlike the TER, a significant difference in NITBUT was found after the instillation of Systane ULTRA (P = 0.01) and Artelac Advanced (P = 0.02). The NITBUT at 10 and 30 min were significantly higher after the instillation of Systane compared with the baseline NITBUT (P = 0.015). No significant difference was found after 20 and 60 min from the instillation of Systane. However, the NITBUT reading was 32% and 19% higher at 20 and 60 min time points, respectively, compared with the baseline NITBUT. A significant increase in NITBUT was found between baseline time and at a time of 10 min (P = 0.017) after the instillation of Artelac. Although no significant difference was observed at other time points, the NITBUT was 21%, 61%, and 16% higher at 20, 30, and 60 min time points, respectively, compared with the baseline time. The mean and standard deviation of NITBUT readings measured at baseline and different time points are shown in Table 2.
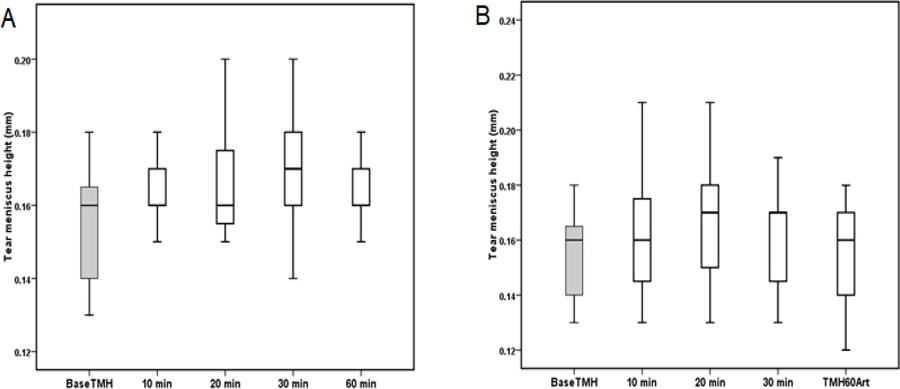
The TMH measured before and after the instillation of Systane ULTRA and Artelac Advanced eye drops are shown in (Fig. 3). The mean TMH at baseline was 0.15 mm. Although an increase in the TMH was found after the instillation of Systane ULTRA and Artelac Advanced, no statistically significant difference was found between the TMH measured before and after instillation of eye drops Table 3.
4. DISCUSSION
This study was conducted to evaluate the efficacy of two different mucomimetic polymer formulations in managing tear film parameters in subjects with dry eyes. The current study found that the tear film evaporation rate was 14% lower than after instillation of Systane ULTRA eye drop. This effect was not observed using Artelac Advanced eye drop where the evaporation rate reading at all-time points remained almost the same as those measured at baseline.
According to the definition and classification subcommittee of the International Dry Eye Workshop, evaporative dry eye is considered one of the major groups of dry eye causes [1]. Controlling the TER is the main role of the lipid layer of the tear film. It has been suggested that the tear lipid layer has two phases (inner polar and outer nonpolar), which work together to maintain healthy, stable tear film [21]. The outer thick nonpolar phase plays a vital role in protecting the tear film from excessive evaporation [21, 22]. However, this function could be affected by several factors, such as Meibomian gland dysfunction, as the Meibomian gland is the main source of tear lipids [23]. Therefore, preserving the tear film lipid layer using eye lubricants is one of the methods used to control the signs and symptoms of dry eye.
The efficacy of HP-Guar formulation in preserving the lipid layer of the tear film is well documented [24, 25]. Improved corneal staining and enhanced tear film stability have been found following the use of tear film supplements containing HP-Guar [26, 27]. Moreover, the ability of Systane to control ocular discomfort scores in patients with dry eyes has been reported [9].
A previous study on lipid layer thickness using interference patterns found that instillation of one drop of Systane eye drop increased the lipid layer thickness by 16% [28]. Therefore, the reduction in evaporation rate observed in this study could be due to the increase in lipid layer thickness. This is in agreement with other studies that found a 13% reduction in tear film evaporation rate 30 min after instillation of a Systane eye drop [29].
Systane ULTRA is a tear supplement containing HP-Guar. Systane also contains polyethylene glycol 400 (0.4%) and propylene glycol (0.13%). It is believed that HP-Guar increases retention and provides a mucin mimic effect that relieves damaged corneal epithelium [11]. HP-Guar is formed by treating a water-soluble plant-derived polysaccharide (guar galactomannan) with propylene oxide [30]. The difference in pH between the bottle and the eye results in the formation of a soft gel layer once Systane is applied into the eye [10]. Moreover, it is believed that HP-Guar increases the retention time of tear supplements in the eye [31]. This could explain the ability of Systane eye drop to enhance the tear film stability that was noted in this study, thus reducing the evaporation rate.
Both tear supplement formulations were successfully managed to improve tear break-up time. In the current study, the tear break-up time was increased up to 61% and 32% following instillation of Artelac Advanced and Systane ULTRA eye drops, respectively. This is in agreement with previous studies that reported an improvement in tear film stability and break-up time with the use of formulations containing HP-Guar and hyaluronic acid [32]. The improvement in tear film stability could improve the quality of vision by reducing the optical aberrations which may be created by tear film instability and irregularities of the corneal surface.
The efficacy of hyaluronic acid in the management of dry eye and ocular surface damage has been well documented. It has been found that hyaluronic acid promotes the corneal wound healing process by stimulating the migration, adhesion, and proliferation of the corneal epithelium [33]. A previous study has reported a significant improvement in the NITBUT at 15, 30, and 60 min time points following the instillation of hyaluronic acid when compared with that when hyaluronic acid was not used [32]. This correlated well with a study that showed an improvement in the NITBUT and reduction in dry eye symptoms among patients with dry eye for up to 6 hours after using sodium hyaluronian eye drops [34]. Sodium hyaluronian has also been found to significantly decrease corneal and conjunctival staining and improve the NITBUT in patients with Sjögren syndrome when used six times daily for 90 days [35].
In previous studies, hyaluronic acid has been shown to have the ability to increase tear volume in patients with dry eye [32]. The improvement in the tear mucous layer resulting from the use of mucomimetic supplements leads to enhancement of tear film stability and tear spread [36]. This could explain the significant increase in the NITBUT (up to 61%) after instillation of Artelac Advanced eye drop.
Hyaluronic acid is a mucoadhesive polymer that has the ability to enhance ophthalmic solution delivery and retention in the eye [37]. It has been shown that antibiotic (ofloxacin and gentamicin) concentration in the cornea and aqueous humor was significantly higher when the antibiotics were viscosified with Hyaluronic acid compared with that with antibiotics alone [38]. Similarly, Ghelardi et al. suggested that hyaluronic acid is considered a promising vehicle for topical ocular drops for its ability to prolong the ocular residence time of eye drops [39].
The current results agree with a study conducted on dry eye subjects (N = 30; 22.14 ± 2.34 years) to investigate the short-term effect of Refresh Plus preservative-free lubricant eye drops on the tear film quality using tear ferning (TF) test. The TF patterns have improved significantly (P = 0.02, Wilcoxon test) after the application of eye drops, and the improvement in tear film quality was maintained for 3 hours [40].
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures were approved by the College of Applied Medical Sciences Ethics Committee, King Saud University, Riyadh, Saudi Arabia.
HUMAN AND ANIMAL RIGHTS
No Animals were used for studies that are base of this research. All human procedures were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All subjects provided written informed consent.
STANDARD OF REPORTING
STROBE Guideline has been followed in this study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.