All published articles of this journal are available on ScienceDirect.
Is Corneal Collagen Cross-Linking Beneficial as an Adjunct to the Conventional Treatment of Bacterial Keratitis?
Abstract
Background:
To evaluate the therapeutic effects of corneal collagen cross-linking CXL as an adjuvant to standard antimicrobial agents in the treatment of bacterial keratitis when compared to treatment with antimicrobial agents alone.
Methods:
This prospective comparative interventional study included 20 eyes of 20 patients with clinical and laboratory evidence of bacterial keratitis who attended the Outpatient Cornea Unit, Ophthalmology Department, Faculty of Medicine in Assiut University Hospital, Assiut, between January 2019 and December 2020.Patients were divided into two groups: group A, treated with CXL using the Dresden Protocol at the EL-Nour Eye Centre, and group B treated with antibiotics alone.
Results:
Group A had ten patients in the age range of 20-80 years (mean age 49.2 years), while that of group B (ten patients) was 19 -70 years (mean age 47.3 years).
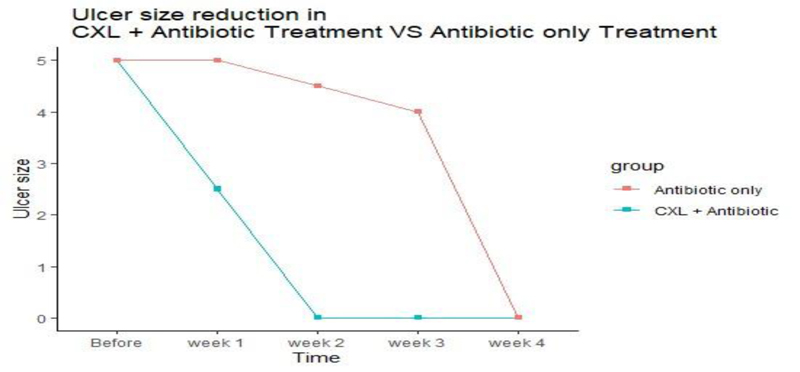
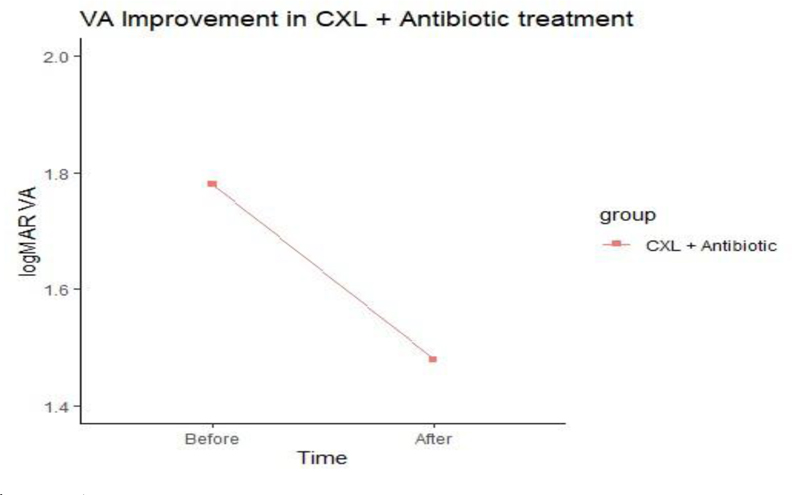
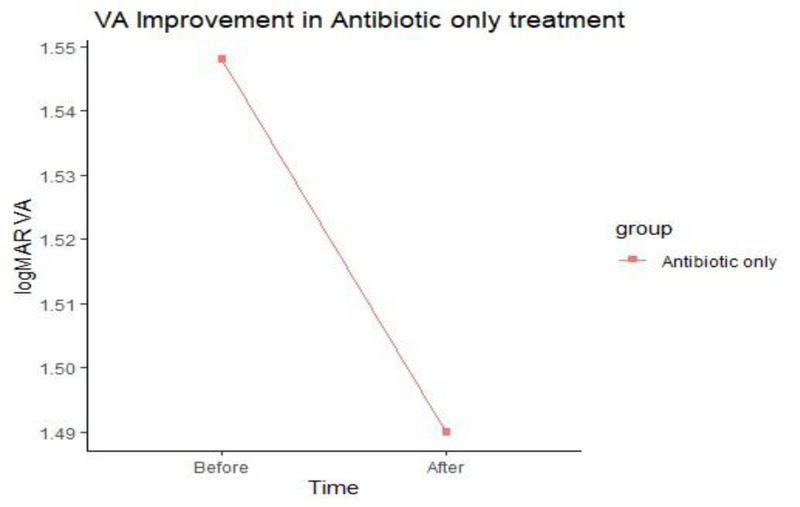
The ulcer sizes started to decrease significantly from week 2 in group A to week 3 in group B. The epithelization time was significantly different between the two treatment groups as reepithelization in 60% of group A cases started at week two, while it began at week three in group B. There was no significant difference in the V/A between the two groups after treatment.
Conclusion:
CXL as an adjunct to topical antimicrobial treatment was more effective in treating bacterial keratitis than conventional antimicrobial therapy alone, as it led to shorter recovery times due to more rapid ulcer healing, resolution of infiltration, and faster symptomatic relief in patients. Despite CXL promoted the ulcer to heal quickly, there was no significant change in V/A before and after CXL or between the CXL with antimicrobial or antimicrobial therapy alone .
1. INTRODUCTION
Microbial keratitis also referred to as the infectious corneal ulcer, is potentially blinding corneal eye disease that has the risk of developing into severe visual deterioration if not treated early [1]. It occurs because of proliferation of bacteria, viruses, fungi, or parasites within the corneal tissue, leading to inflammation and tissue destruction [2, 3]. Risk factors include contact lens use, recent ocular operation, ocular trauma, pre-existing ocular surface disease, dry eye, lid deformity, sensational corneal dysfunction, and prolonged use of topical steroids or systemic immunosuppression [4-6].
The ideal management of microbial keratitis is a challenge and a topic of ongoing research. Topical broad-spectrum antibiotics [7], topical steroids [8], and amniotic membrane transplantation [9] have been used to eliminate the infection or decrease the inflammatory response of the cornea. However, antimicrobial treatments are subject to microorganism sensitivity and resistance [10, 11].
Corneal collagen cross-linking (CXL) is a technique that changes the collagen matrix of the corneal stroma by creating riboflavin ultraviolet A (UVA)-induced cross-links. The process is based on riboflavin as a photosensitizer, which, when triggered by UVA at 365 or 370 nm, generates reactive oxygen species, giving rise to cross-links in the corneal stroma. It is used to treat corneal ectasia in progressive keratoconus and pellucid marginal degeneration, as well as post-refractive surgery ectasia [12, 13].
Recent studies suggest that CXL may also be helpful in non-metabolic corneal disorders, especially corneal edema, as cross-linking creates a compact stroma with less space for fluid accumulation [14, 15]. Its application in bacterial keratitis is due to altered collagen properties caused by photoactivated riboflavin, which stabilizes the corneal stroma by stiffening it and increases its resistance to enzymatic degradation caused by microorganisms, which stops the progression of corneal melting [16, 17]. When illuminated, riboflavin exhibits antimicrobial properties because it causes the oxidation of nucleic acids of microorganisms [18]. Thus, CXL can be considered an antimicrobial treatment not subject to microorganism sensitivity or resistance and has the benefit of halting the enzymatic melting process [19].
This prospective comparative interventional study aimed to evaluate the therapeutic effects of corneal CXL as an adjuvant to standard antimicrobial agents in the treatment of bacterial keratitis when compared to treatment with antimicrobial agents alone. We compared the rate of reduction of ulcer size and resolution of infiltration, as well as improvement in visual acuity and symptomatic relief.
2. MATERIALS AND METHODS
2.1. Study Design
This study included 20 eyes of 20 patients with clinical and laboratory evidence of bacterial keratitis who attended the Outpatient Cornea Unit, Ophthalmology Department, Faculty of Medicine in Assiut University Hospital, Assiut, between January 2019 and December 2020. Assuit University's Institutional Review Board and Ethics Committee approved this study, which followed the Declaration of Helsinki's principles. Each patient was informed about the nature of the study, CXL details, its intra and postoperative complications and prognosis. A written informed consent was obtained from each patient enrolled in the study.
A detailed history was recorded, and a complete eye examination, including visual acuity V/A measurement and slit lamp biomicroscope, was performed for each patient. Corneal ulcer size was measured by a special caliper . Ulcer location, time of onset, depth and margin of infiltration, possible associated complications, and treatment were recorded.
Patients with a corneal thickness less than 400 μm (measured by Ant. Segment OCT), corneal perforation or scar, coexisting ocular pathology or ocular surface disease, autoimmune or collagen vascular disease, or pregnant women were excluded from the study.
Ulcer size between 2 to 7 mm was included in the study as ulcer less than 2 mm was amenable to healing and more than 7mm was extensive ulcer.
After obtaining patients' consent to surgery, we divided them into two groups as masked randomization:
Group A: Ten eyes of 10 patients were treated with CXL in addition to standard antimicrobial treatment.
Group B: Ten eyes of 10 patients were treated with standard antimicrobial treatment only. One author examined and followed up all patients.
2.2. Methods
Standard antimicrobial therapy was started as monotherapy in both treatment groups with fourth-generation fluoroquinolone (Vigamox, Alcon Laboratories, Inc., Fort Worth, TX, USA) eye drops that were administered each hour per day, and subsequently modified based on clinical response, as well as culture and antibiotic sensitivity results. Cyclopentolate hydrochloride ophthalmic solution (1%; Swixolate) was administered three times daily.
2.2.1. Surgical Technique of CXL in Group A
All procedures were performed at the EL-Nour Eye Centre, Assiut, by Professor Mohamed Saad Abd-Elrahman, between January 2019 and December 2020. The standard Dresden protocol was used. First, the cornea was anaesthetized with topical tetracaine (0.1%) twice, with an interval of 5 min, just before the procedure. Eyelashes were isolated using a drape and a speculum. An 8-mm zone of the corneal epithelium, including all microbial infiltrates, was removed using a thin blunt metal (Hockey knife), and 0.1% riboflavin in 20% dextran 500 ophthalmic solution (MEDIOCROSSTM Medio- Haus Medizinprodukte GmbH, Behrens brook 6, D-24214 Neudorf) was administered every three min for 30 min. The cornea was then exposed to UV-A rays (365 nm) in an optical zone of 8 mm for 30 min with an irradiance of 3 mW/cm2 (UV-XTM; Peschke Meditrade, Cham, Switzerland). During the procedure, the cornea was moistened with 0.1% riboflavin and tetracaine. After irradiation, a therapeutic soft contact lens was placed for 4 days. All patients received topical antibiotics and artificial tear drops until epithelial healing was observed.
After treatment with CXL, the patients were examined daily in the first week and then weekly for up to 4 weeks to evaluate response to treatment, re-epithelialization, and time taken to resolve stromal infiltrate. The treatment was considered successful if rapid epithelialization occurred with decreased stromal infiltration within 2 weeks of CXL. If there were no signs of improvement, or the ulcer worsened after 4 weeks, the treatment was considered a failure.
2.3. Follow Up
Follow-up appointments for group A were scheduled daily in the first week and then weekly for up to 4 weeks, while those for group B were scheduled weekly for up to 4 weeks.
Our primary outcome measures were reduction in ulcer size (reduction of 20 percentage of ulcer size was consider significant) and resolution of infiltration. The time taken for complete epithelialization, improvement of V/A, and relief of symptoms were the secondary outcome measures.
Assessment of the efficacy of treatment in both groups was done by using:
(1) Photography before the treatment began and then at each weekly follow-up appointment, for up to 4 weeks.
(2) Recording signs of improvement (V/A; ulcer: decrease in size and infiltration, epithelization, and increased vascularization).
(3) Assessment of relief of symptoms (pain, redness, lacrimation, and photophobia).
This prospective comparative interventional study was performed in accordance with the tenets of the Declaration of Helsinki and was reviewed and approved by the Assiut University Institutional Review Board. Written informed consent was obtained from all patients before enrollment in the study, following a discussion about the nature of the study and the risks and benefits of participation.
2.4. Statistics
2.4.2. Analytical Statistics
- All categorical variables were expressed as count and percent.
- The tests were performed after the normality was tested with the Shapiro-Wilk normality test, so the numerical data that were not normally distributed were expressed as median and interquartile range (IQR), and the data that were normally distributed were expressed as mean and standard deviation (SD). P-values < · 0.05 were considered significant with a 95% confidence interval.
- V/A, ulcer size, and infiltration were described as median and interquartile range (IQR) and compared between the two treatments using the Kruskal-Wallis rank-sum test and Dunn’s multiple comparisons test. The ulcer sizes, before and at the one-week interval after treatment, were expressed as mean and SD compared between two treatments using the Welch two-sample t-test. All categorical variables were compared with the Fisher exact test.
3. RESULTS
3.1. Descriptive Analysis of the Study Groups
Group A had ten patients in the age range of 20-80 years (mean age 49.2 years), while the age of group B (ten patients) was 19 -70 years (mean age 47.3 years). Of all participants, 40% were female. The left eye was infected in 70% of patients. The causative bacteria were Staphylococcus aureus in 35% of cases, Pseudomonas in 30%, Streptococcus in 30%, and Staphylococcus epidermidis in the remaining cases. For both groups, the infection site was paracentral in 50% of the eyes, central in 40%, and peripheral in 10%.” The mean ulcer size in both groups was 5.4. The median of infiltration in group A was 0.5, and that in group B was 0.4. The median V/A before therapy in group A was 1.8. and that in group B was 1.6. There were no significant differences between the two pre-treatment groups (P >0.05 from all descriptive variables).
3.2. Results Analysis of the Study Groups
3.2.1. Ulcer size
The ulcer sizes started to decrease significantly from week 2 in group A to week 3 in group B. At the first, second-, and third-week intervals, the ulcer size in group A was significantly smaller than that in group B (P = 0.006, 0.004, and 0.002, respectively) (Table 1, Fig. 1). Reduction of 20 percentage of ulcer size was considered significant.
3.2.2. V/A
No significant difference in V/A was noted between the two groups after treatment (Figs. 2 and 3).
| Results | Group A | Group B |
Total n (%) |
P-value |
| V/A after treatment (Median, IQR) |
1.5 (1.3 to 1.5) |
1.4 (1.1 to 1.7) |
0.759 | |
| Ulcer size after week 1 (Mean, SD) |
3.0 (1.6) |
5.1 (1.4) |
0.006 | |
| Ulcer size after week 2 (Median, IQR) |
0.0 (0.0 to 1.0) |
4.5 (4.0 to 5.0) |
0.004 | |
| Ulcer size after week 3 (Median, IQR) |
0.0 (0.0 to 0.0) |
4.0 (3.0 to 4.0) |
8(40% | 0.002 |
| Ulcer size after week 4 (Median, IQR) |
0.0 (0.0 to 0.0) |
0.0 (0.0 to 3.8) |
12(60%) | 0.284 |
| Epithelization | 0.001 | |||
| Not Yet | 2 (20.0) | 4(40.0) | 6 (30%) | |
| W2 | 6 (60.0) | 0 (0.0) | 6 (30%) | |
| W3 | 2 (20.0) | 0(0.0) | 2 (10%) | |
| W4 | 0(0.0) | 6(60.0) | 6 (30%) | |
| Outcome | 0.628 | |||
| Improvement | 8 (80.0) | 6 (60.0) | 14 (70%) | |
| No Improvement | 2(20.0) | 4(40.0) | 6 (30%) |
All P-values of numerical variables were based on the Kruskal-Wallis rank-sum test.
Except for the ulcer size after week 1, P-value was based on the Welch two-sample test.
3.2.3. Ulcer epithelization time
The epithelization time was significantly different between the two treatment groups as reepithelization in 60% of group A cases started at week two, while it began at week three in group B (P = 0.001) (Figs. 4 and 5).
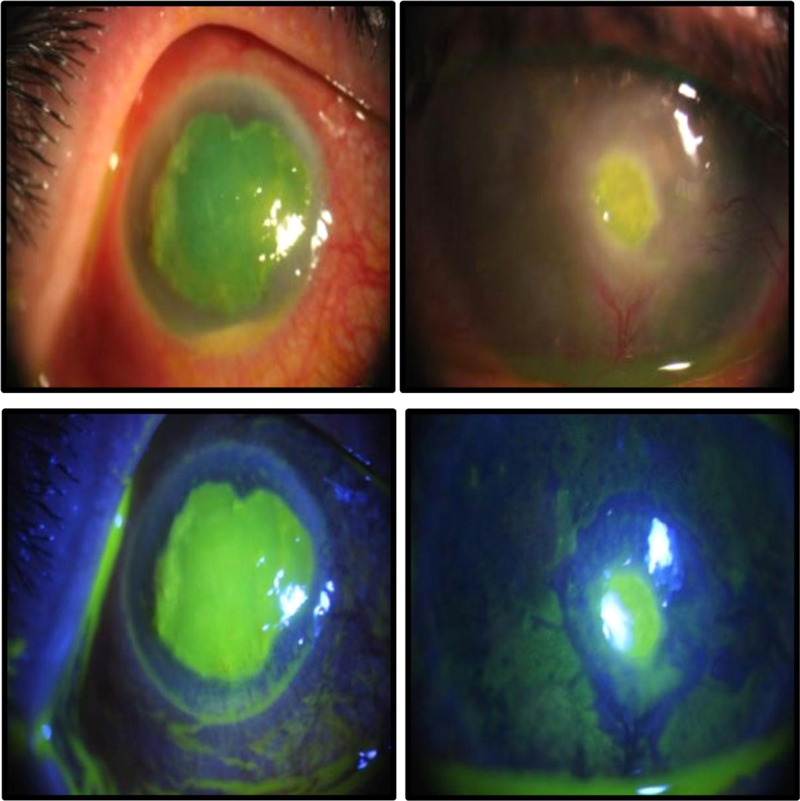
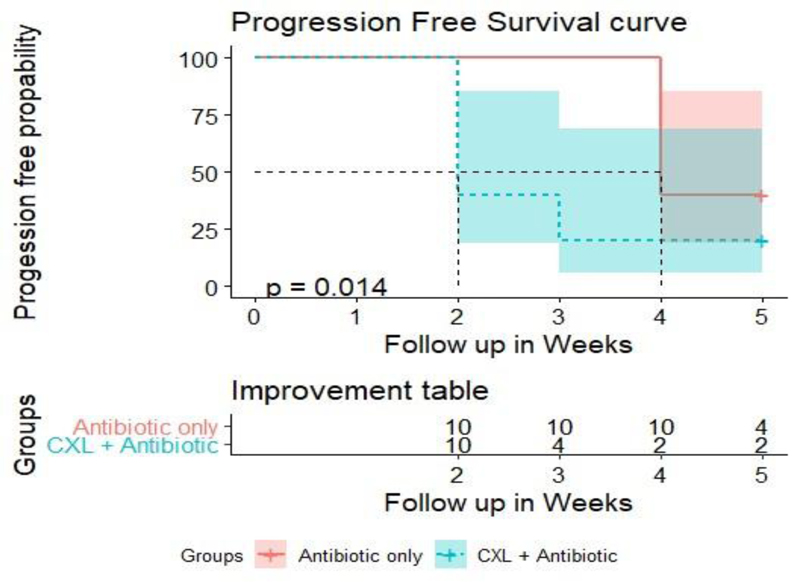
Progression-free survival curve analysis revealed that improvement of signs and symptoms in 50% of cases occurred after 2 weeks in group A and after 4 weeks in group B (P = 0.014) (Fig. 6).
4. DISCUSSION
Infectious keratitis, with an estimated incidence of 1.4 million cases per year, can lead to ulceration, corneal melting, and perforation, causing permanent visual loss if left untreated [20, 21]. Although broad-spectrum and fortified antibiotics remain the primary treatment for bacterial keratitis, antimicrobial resistance, and the lack of new antimicrobial drugs are emerging problem [22, 23]. Antimicrobial resistance and the lack of new antimicrobial drugs is an emerging problem that can potentially have adverse effects, such as ulceration, corneal melting, and perforation, causing permanent visual loss [24, 25]. Recent studies suggest that CXL may have applications in the treatment of bacterial corneal ulcers, owing to its ability to halt corneal melting and the antimicrobial properties of photoactivated riboflavin [26, 27]. This study was conducted to determine whether riboflavin/UV-A corneal CXL in conjunction with standard antimicrobial agents was more effective in treating bacterial keratitis than treatment with antimicrobial agents alone.
Of a total of 20 eyes that were treated in the course of this study, we noticed that the ulcer sizes in the eyes that received CXL were significantly lower at the first, second, and third week intervals when compared to those who received antimicrobial treatment alone. The time taken for complete epithelialization in 60% of the cases was almost double in patients who received antimicrobial treatment alone compared to those who received CXL with antimicrobial.
Improvement, in 50% of the cases, occurred in two weeks in patients treated with CXL, while it occurred in four weeks in those who received antimicrobial treatment alone. Iseli et al. [28] first used CXL for treating infectious keratitis in 2008 and reported a halt of the corneal melting process and a decrease in infiltration size in 4 of 5 patients. Spoerl et al. [16] reported cessation of corneal melting and decrease in infiltrate sizes in four out of five cases after CXL therapy was used to treat antibiotic-resistant infectious keratitis. In a non-randomized clinical trial, Makdoumi et al. [29] observed that all patients initially responded to CXL as a single first-line treatment without any antimicrobial drugs,
while only 12.5% of these patients needed additional antibacterial treatment. In 2014, Said et al. [30] reported no significant differences in corneal healing times and final visual outcomes between patients who received CXL in addition to medical therapy and those who received medical therapy alone. However, they noted that patients who received CXL had larger mean diameters of corneal ulcers and fewer late complications, such as corneal perforation and recurrence of infection. Panda et al. [31] reported a halt in the progression of corneal melting in all cases treated with CXL. Skaat et al. [32] reported resolution of all signs of infection and inflammation in five of six cases of severe refractory infectious keratitis within 1-2 weeks of treatment with CXL. However, one patient required penetrating keratoplasty. Sorkhabi et al. [33] concluded that CXL is a viable therapeutic option for treating corneal ulcers and can be used as an adjuvant in resistant cases.
The findings in our study are in agreement with those of the study by Bamdad et al. [34], which was a randomised comparative clinical trial in 2015 that reported accelerated infiltration resolution, better epithelial healing, and shorter treatment time in cases of moderate bacterial keratitis treated using CXL with medical treatment when compared to those receiving medical treatment alone.
CONCLUSION
In conclusion, our study suggests that CXL as an adjunct to topical antimicrobial treatment was more effective in treating bacterial keratitis than conventional antimicrobial therapy alone, as it led to shorter recovery times due to more rapid ulcer healing, resolution of infiltration, and faster symptomatic relief in patients. Although the CXL+ antimicrobial group had a faster healing rate, there was no significant difference in V/A pre and postoperatively. Furthermore, there was no difference in final V/A between the two study groups.
A study on a larger scale, including a greater number of eyes, could provide a better understanding and reduce the margin of error. The limitations of our study are the cost of the surgery and patient unwillingness created obstacles in our original study plan. Future studies could explore the effectiveness of CXL in infectious keratitis caused by microorganisms other than bacteria.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Assuit University's Institutional Review Board and Ethics Committee approved this study.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Each patient was informed about the nature of the study, CXL details, its intra and postoperative complications and prognosis. A written informed consent was obtained from each patient enrolled in the study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


