RESEARCH ARTICLE
Optical Coherence Tomography Angiography Findings in β-Thalassemia Patients in Two Age Groups
Hany Mahmoud1, *, Eman H. Salama2, Asmaa A. Abdel-baset3, Mahmoud Gaber3, Eman Mohamed Fahmy4, Dalia Tohamy5, Mohamed Anbar1, Engy M. Mostafa1
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
E-location ID: e187436412211110
Publisher ID: e187436412211110
DOI: 10.2174/18743641-v16-e221115-2022-13
Article History:
Received Date: 18/4/2022Revision Received Date: 5/10/2022
Acceptance Date: 20/10/2022
Electronic publication date: 30/12/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose:
β- thalassemia major causes hypoxia, which affects the retinal nerve fiber layer (RNFL), causing apoptosis. The frequent blood transfusion in transfusion-dependent thalassemia (TDT) accumulates ferritin, causing more damage. Using Optical Coherence Tomography (OCT) and Optical Coherence Tomography Angiography (OCTA), we aim to compare these changes in Youngs and adults.
Methods:
In this cross-sectional non-randomized comparative study, 50 TDT patients were included: 28 were under 18 years (group1) and 22 were above 18 years (group2). They were subjected to hematological examination and ophthalmological examination, including OCT and OCTA. Then, the data were collected and analyzed.
Results:
There was a statistical difference between the age in both groups (p=0.01). The two groups did not differ in sex distribution as well (p=0.085). All hematological parameters showed fewer values in (group 2) with a statistically significant difference in Serum ferritin, showing a marked increasing difference. There was a statistical difference between all Macular parameters and peripapillary quadrantal RNFL thickness of both groups (except for the C/D ratio), with higher values in (group 1). A moderate or strong positive correlation was found between all retinal parameters except for the C/D ratio and Hb level. A moderate or strong positive correlation was found between all retinal parameters except for the C/D ratio and serum ferritin. Moreover, there was a moderate to strong negative correlation between all retinal parameters except for the C/D ratio and frequency of blood transfusion.
Conclusion:
patients above 18 years (group2) are more affected by more RNFL thinning and vascular density changes.
1. INTRODUCTION
β-thalassemia is a myriad of autosomal recessive hematological disorders that are prevalent worldwide yet show an increasing percentage of cases in the Mediterranean area [1, 2]. It results from a mutation in chromosome 11 affecting the β- globin chain production [1]. The disease severity varies from mild or asymptomatic anemia to severe anemia according to the degree of affection of the β- globin chain affection [3], which causes the red blood cells to suffer premature damage from oxidative damage of the cell membrane in the bone marrow.
β-thalassemia major is a severe form of the disease, which requires regular blood transfusion within the first year of life as patients become dependent on it (TDT) [4]. Consequently, the accumulation of ferritin occurs in body organs [5]. Due to the inability of the body to eliminate ferritin, chelating agents are used as a mandatory adjuvant treatment to promote its excretion. Both thalassemia and anti-ferritin chelating agents can cause ocular affection in the form of retinal pigment epithelium (RPE) mottling, RPE degeneration, angioid streaks, venous tortuosity, night blindness, visual field defects, decreased visual acuity, color vision abnormalities, and acute visual loss [6].
Optical coherence tomography (OCT) has revolutionized retinal imaging techniques because of its speed, non-invasiveness, and microscopic assessment of the retinal layers and optic nerve. The advent of OCT angiography (OCT-A) has added the ability to investigate vascularity at both the superficial capillary plexus (SCP) and deep capillary plexus (DCP) [7, 8].
In this study, we aim at exploring the effect of the duration and frequency of TDT on the macular vessel density (superficial and deep plexus) and peripapillary retinal nerve fiber layer (RNFL) using OCT and OCT angiography.
2. PATIENTS AND METHODS
2.1. Ethical Consideration
This study is a cross-sectional non-randomized comparative study that was approved by the Sohag Faculty of Medicine Ethical Committee, Egypt: IRB number: Soh-med-21-04-37. It is adherent to the tenants of Helsinki. Written consent was taken from patients and their legally authorized representatives (parents). This study was conducted in the Ophthalmology Department in collaboration with the Pediatric, Internal Medicine, and Clinical Pathology Departments.
2.2. Study Population
The study included 50 patients with β-thalassemia major with thalassemia-dependent transfusion (TDT) and on iron chelating agents. (Group 1) included 56 eyes of 28 patients <18 years old, and (Group 2) included 44 eyes of 22 patients > 18 years old.
2.3. Exclusion Criteria
Any ocular disease except for minor refractive errors (<2D) or previous ocular surgery and any systemic disease other than thalassemia were excluded. Unacceptable image quality photos were discarded as well.
All patients were subjected to full hematological examination and full ophthalmological examination: OCT and OCT angiography using Angiovue optical coherence tomography angiography (Optovue RTVue XR Avanti, Optovue Inc., Fremont, CA) by one experienced observer. The peripapillary RNFL thickness was evaluated in four sectors, including superior, inferior, temporal, and nasal, along with the ganglion cell complex (GCC). Macular vessel density superficial capillary plexus layers were also reported.
2.4. Statistical Analysis
Statistical analyses were done using the Statistical Package for the Social Sciences (IBM® SPSS® software version 22.0) (SPSS, Inc., Chicago, IL, USA). Eyes were taken as individual units of analysis in our study. Quantitative data were represented as mean (±) standard deviation, and qualitative data were presented as number and frequency percentage.
Differences between groups in age and sex were analyzed by the Mann-Whitney U-test for continuous measures and the Fisher exact test for categorical measures. © Correlation was done by the Spearman test, the strength of correlation was defined as negligible (r < 0.20), weak (0.21 < r <0.40), moderate (0.41 < r < 0.70), or strong (0.71 < r < 1).
3. RESULTS
The clinical characteristics of both groups are given in Table 1.
Patients in (group 1) had a mean age of (10.64+0.30) years old, with twenty eyes (71.4%) for males and eight eyes (28.6%) for females. Patients in (group 2) had a mean age of (20.00+0.98) years old with ten eyes (45.45%) for males and twelve eyes (54.55%) for females. There was a statistical difference in age between both groups (p=0.01), but the two groups did not differ in sex distribution (p=0.085).
As the number of blood transfusions were higher in (group 2) due to prolonged years of the disease (p=0.04), all hematological parameters showed fewer values in (group 2) with a statistically significant difference, with Serum ferritin showing a marked increase difference (Fig. 1).
| - | Group 1 (Mean+SD) | Group 2 (Mean+SD) | p-value |
|---|---|---|---|
| WBC Cell/microlitre |
21.875+12.12 Range (5.72-27.48) |
14.127+13.75 Range (5.4.38-22.19) |
0.001 |
| MCV Femtolitre/cell |
58.57+8.46 Range (50.35-66.89) |
57.82+9.38 Range (48.84-64.89) |
0.05 |
| PLt Cell/microlitre |
550.79+257.67 Range (320.28-700.92) |
535.82+215.16 Range (301.11-678.28) |
0.01 |
| Hb (g/dl) | 6.579+1.70 Range (5.462-7.75) |
7.991+1.62 Range (5.987-7.874) |
0.01 |
| S Ferritin (ng/mL) | 566.75+249.54 Range (340.28-690.37) |
1745.59+487.14 Range(1460.42-1980.82) |
<0.001 |
| Frequency of blood transfusion (number of times) | 100.54+8.7 Range (92.28-109.92) |
147.45+11.25 Range (139.58-158.88) |
0.04 |
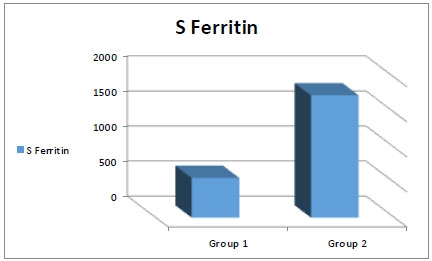 |
Fig. (1). S. ferritin in both groups. |
| Group 1 (mean+SD) | Group 2 (mean+SD) | P value | |
|---|---|---|---|
| CMT μm (micrometer) |
232.71+24.76 Range (207.12-249.58) |
189.32+8.58 Range (179.87-208.67) |
<0.001 |
| VD SCP % Percentage |
22.29+7.79 Range (16.17-28.94) |
16.45+7.91 Range (12.95-22.79) |
0.019 |
| VD DCP % Percentage |
54.14+10.89 Range (45.14-64.42) |
47.00+10.84 Range (40.87-58.68) |
0.029 |
| Superior RNFL μm (micrometer) |
108.71+9.16 Range (100.33-118.39) |
102.73+16.79 Range (85.87-117.85) |
0.012 |
| Inferior RNFL μm (micrometer) |
107.29+8.52 Range (99.12-115.27) |
99.00+10.15 Range (89.12-108.57) |
0.004 |
| Temporal RNFL μm (micrometer) |
79.86+7.23 Range (70.22-86.87) |
75.39+9.36 Range (67.12-85.71) |
0.008 |
| Nasal RNFL μm (micrometer) |
82.03+6.94 Range (76.15-89.77) |
79.36+6.91 Range (75.33-87.84) |
0.006 |
| C/D Ratio |
0.31+0.12 Range (0.22-0.42) |
0.27+0.12 Range (0.21-0.39) |
0.35 |
| Avg GCC μm (micrometer) |
101.00+5.21 Range (97.48-107.72) |
91.64+5.19 Range (86.25-98.49) |
<0.001 |
The Macular parameters and peripapillary quadrantal RNFL thickness of both groups are shown in (Table 2 and Fig. 2). There was a statistical difference between all parameters (except for the C/D ratio), with higher values in (group 1).
| - | HB | S Ferritin | Frequency of Blood Transfusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | Group 1 | Group 2 | Group 1 | Group 2 | Group 1 | Group 2 | ||||||
| - | r | P value | r | P value | r | P value | r | P value | r | P value | r | P value |
| Macular thickness | 0.42 | 0.05 | 0.61 | 0.05 | -0.48 | 0.04 | -0.72 | 0.03 | -0.51 | 0.02 | -0.68 | 0.09 |
| VD SCP | 0.49 | 0.04 | 0.82 | 0.01 | -0.51 | 0.05 | -0.90 | 0.04 | -0.48 | 0.05 | -0.73 | 0.002 |
| VD DCP | 0.61 | 0.008 | 0.58 | 0.004 | -0.54 | 0.04 | -0.73 | 0.006 | -0.62 | 0.02 | -0.69 | 0.01 |
| Superior RNFL | 0.53 | 0.03 | 0.48 | 0.03 | -0.61 | 0.008 | -0.69 | 0.03 | -0.55 | 0.003 | -0.77 | 0.03 |
| Inferior RNFL | 0.51 | 0.05 | 0.39 | 0.001 | -0.69 | 0.002 | -0.63 | 0.04 | -0.44 | 0.004 | -0.68 | 0.001 |
| Temporal RNFL | 0.34 | 0.05 | 0.75 | 0.01 | -0.43 | 0.02 | -0.62 | 0.009 | -0.71 | 0.03 | -0.81 | 0.009 |
| Nasal RNFL Average RNFL |
0.29 0.47 |
0.04 0.05 |
0.52 0.48 |
0.03 0.04 |
-0.34 -0.51 |
0.01 0.004 |
-0.59 -0.54 |
0.04 0.05 |
-0.54 -0.39 |
0.01 0.04 |
-0.74 -0.64 |
0.01 0.03 |
| C/D | 0.12 | 0.18 | 0.11 | 0.14 | -0.25 | 0.51 | 0.05 | 0.42 | 0.09 | 0.36 | 0.66 | 0.49 |
| Avg GCC | 0.46 | 0.04 | 0.59 | 0.004 | -0.56 | 0.02 | -0.79 | 0.001 | -0.59 | 0.004 | -0.85 | 0.007 |
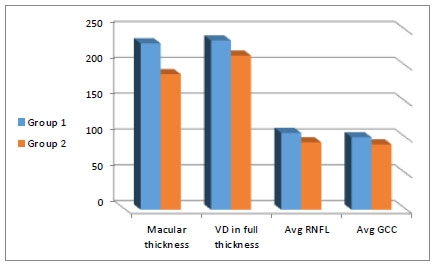 |
Fig. (2). OCT and OCT-A parameters in both groups. |
3.1. Correlation
Using the Spearman correlation test, there was a moderate or strong positive correlation between all retinal parameters except for the C/D ratio and Hb level. That is, the higher level of Hb is, the higher retinal parameters become, and vice versa.
There was a moderate or strong negative correlation between all retinal parameters except for the C/D ratio and s ferritin. In other words, the higher s ferritin is, the lower the retinal parameters become, and vice versa.
There was a moderate to strong negative correlation between all retinal parameters except for the C/D ratio and frequency of blood transfusion. That is, the more frequent blood transfusion is, the lower retinal parameters become, and vice versa (Table 3).
4. DISCUSSION
β-thalassemia major can cause lens opacification, decreased contrast sensitivity, visual field defects, and electrophysiology changes in the retina in the form of reduction in a-wave and b-wave in Electroretinography (ERG), and adecrease in the Arden ratio in Electrooculography (EOG) [9].
The effect of β-thalassemia major on the retina has been vastly reported in the form of pseudoxanthoma elasticum-like findings.
However, its ischemic effect on the retinal microvasculature detected by OCT-A is still in progress [10].
Retinal nerve fibers RNFL are the unmyelinated extensions of the ganglion cells. They are supplied by the central retinal artery. Moreover, choroidal vasculature provides blood supply to the outer retina. Several studies showed that ischemic conditions lead to apoptosis of RNFL in thalassemia [11]. Another degrading factor in thalassemia major patients. The RNFL is a frequent blood transfusion in TDT patients with resultant accumulation of ferritin [12]. Iron overload causes damage to the neuronal cells due to oxidative stress and free radicals effects [13]. The chelation therapy could lower the serum ferritin with less organ damage.
The toxic effect of iron overload leads to endothelial toxicity with resultant decreased vascular perfusion and increased ischemia [14].
Our results showed a greater decrease in the thickness of the RNFL in (group 2) than in (group 1). This finding might be related to the chronicity of anemia that leads to hypoxia, as reported by previous studies [15]. Also, hypoxia may influence retinal and choroidal circulation [16].
In this study, patients older than 18 years showed fewer vessel density in the macular area (both superficial and deep plexuses). This finding might be attributed to chronic hypoxia or even the increased number of blood transfusion frequency in the older age group with more accumulation of ferritin, which causes increased radicals and oxidative stress. [17, 18]
There is a significant difference in the hematological parameters in both groups. The correlation study detected a significant correlation between HB, serum Ferritin, and all measured retinal parameters except for the macular thickness. This result is consistent with the idea of the effect of ischemia on the retinal vasculature and RNFL.
The limitation of this study was the lack of a normal control group, yet we aimed to detect the effect of the prolonged time of blood transfusion on retinal tissue. Further studies would be appropriate to compare the variants of β-thalassemia (major and minor).
The difference in age groups, however, is a method of classification to create two comparative groups but it could be a limitation.
OCT and OCTA proved to be useful in detecting microvascular changes in the cases of the β-thalassemia major. They helped improve our knowledge about the effects of ischemia [19]. This protocol can be applied to other hypoxic-causing diseases to widen our understanding of the retinal response.
LIST OF ABBREVIATIONS
| TDT | = Transfusion-dependent thalassemia |
| OCT | = Optical coherence tomography |
| OCTA | = Optical coherence tomography angiography |
AUTHORS' CONTRIBUTION
All authors collected the study data, wrote, revised, and approved the manuscript. Hany Mahmoud and Engy M. Mostafa made the study design, did the statistical analysis, collected the study data, and wrote, revised, and approved the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Sohag Faculty of Medicine Ethical Committee, Egypt: IRB number: Soh-med-21-04-37.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research paper. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants.
STANDARDS OF REPORTING
STROBE guideline has been followed in this study.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study is available upon reasonable request from the corresponding author [H.M].
FUNDING
None
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.







