RESEARCH ARTICLE
Refractive Error and Ocular Biometry Among Young adults From Cuiabá, Brazil
Celso Marcelo Cunha1, *
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187436412212300
Publisher ID: e187436412212300
DOI: 10.2174/18743641-v17-e230109-2022-33
Article History:
Received Date: 27/6/2022Revision Received Date: 9/12/2022
Acceptance Date: 9/12/2022
Electronic publication date: 02/02/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:
To investigate the distribution of refractive errors and their association with ocular biometric data, family history, and environment variables in medical university students of first to six semesters of UNIVAG - MT.
Methods:
A general ophthalmological exam was performed including corneal topography and ocular optical biometry. Lens power has calculated by Bennett and Rozema’s formula. A questionnaire regarding a family history of myopia and lifestyle visual activities was applied. Only university students with normal ophthalmological exams were included. Statistical significance was considered at the level of p<0.05.
Results:
One hundred twenty-eight students were selected, whereas the other 13 were excluded. The mean age was 21.28 ± 2.18 years. Forty-four (34.4%) participants were males. Regarding refractive errors, 18 (14.1%) were hyperopic, 41 (32%) were emmetropic, 61 (47.7%) were myopic, and 8 (6.3%) were high myopic. The mean and standard deviations of spherical equivalents, axial lengths, keratometries (K1 and K2), and lens powers were -1.27 ± 2.21 D, 24.17 ± 1.07 mm, 42.90 ± 1.25 D, 43.94 ± 1.37 D, and 22.62 ± 1.73 D, respectively. In relation to the family history of myopia, one parent was myopic in 28 (50%) of the subjects, and both parents were myopic in 7 (12.5%) subjects. The average of hours per week spent outdoors were 5.82 +/- 5.80 hs. and spent 2.18 +/- 2.37 hs. at sports activities at night.
Conclusion:
Myopia was the most frequent refractive error among the medical university students subject of this study, and was approximately three times higher than those reported for other samples of the Brazilian population. There was a positive correlation between refraction errors and axial length.
1. INTRODUCTION
Myopia occurs in the eyes in which the image is focused in front of the retina. The focusing location of the image inside the eyeball depends on three optical components: corneal curvature, crystalline power and axial length (AL) [1]. The prevalence of myopia varies depending on region, ethnicity, and age group, and it is more prevalent in industrialized cities with higher education status [2]. In Asian countries, myopia has sharply increased [3, 4]. Other parts of the world, such as the United States of America, have also reported a significant increase in prevalence in recent decades [5]. In Brazil, a country of continental dimensions, there are few studies, even in localized populations, which estimate prevalence around 13% in the general population, but there is a lack of current epidemiological data to estimate the real situation [2, 6, 7]. It is expected that in the coming decades there will be a higher myopia prevalence globally [8].
Myopia routinely develops in schoolchildren but may also appear in young people or adults [2]. The earlier myopia onsets, the greater will be the final myopic power expected in adulthood [9, 10]. Myopia progression is more commonly produced by the increase of AL than for changes in the other optical components [11, 12]. In fact, the measurement of AL has been considered the most accurate way to monitor myopia progression [13].
Emmetropia, by definition, is the ocular refractive state of the eye in which the image is focused on the retina when accommodation is relaxed. The refractive error is between -0.50 D and +0.50 D. Myopia is the refractive ocular condition with the spherical equivalent (SE) ≤ -0.50 D in both eyes. According to SE power, the World Health Organization classifies myopia as [8]:
-Low: > -3 D;
-Medium: -3 to -5 D;
-High: ≤ -5 D.
Patients with high myopia, and consequently, AL ≥ 26 mm, have higher risks of decreased visual acuity in adulthood due to myopic maculopathy, retinal detachment and glaucoma [14, 15].
Although the growth of AL in the population from childhood to young age has been well studied, just a few studies have been reported in young adulthood [10, 11, 16]. Late adolescents are understood as the population between 17 and 19 years old, and young adults, between 20 and 24 years old, according to the World Health Organization [17].
The aim of this study was to evaluate the distribution of ametropias and ocular biometric data and their relationship by gender and family history of myopia in a late adolescent to adult population, represented by subjects of medical university students from Centro Universitario de Várzea Grande, Várzea Grande, MT, Brazil (UNIVAG-MT).
2. MATERIALS AND METHODS
This paper has reported the baseline cross-sectional data of a planned prospective study. All the medical students in their first to sixth semesters at UNIVAG (classes 2018-1, 2018-2, 2019-1, 2019-2, 2020-1, 2020-2), ages 17 to 30, were invited. Subjects were evaluated at the ophthalmology outpatient clinic at Oftalmocenter Santa Rosa in Cuiabá, MT, Brazil.
An ophthalmological examination was conducted with the assessment of visual acuity, the measurement of static refraction with the use of an autorefractor (Canon®, USA) under cycloplegia, with prior administration of 0.5% proparacaine, followed by one application of 1% cyclopentolate eye drops, and two applications of 1% tropicamide, one drop each, with 5-minute intervals between drops. Biomicroscopic examination of the anterior segment was performed using a slit lamp, tonometry (Tono-pen®, USA), cover test, corneal topography, and optical biometry (Lenstar LS900®, Haag-Streit, USA). Lens power was measured indirectly with Bennett and Rozema’s formula using the cycloplegic refraction, K1, K2, anterior chamber depth, lens thickness and AL [18].
The selected students answered a questionnaire about outdoor activities, the history of their myopia onset, and parents' refractive history. All participating students were oriented about the study and those who agreed to participate signed a written informed consent. The study was registered in the Plataforma Brasil Program – CAAE: 40738620.1.0000.5692.
University students with corrected visual acuity ≥ 0.66 in both eyes and with a normal ophthalmologic examination, were included. University students with associated ocular pathologies, with incomplete data, those who did not answer the questionnaire, those with astigmatism ≥ 2 D or topographic irregular astigmatism, allergic to any cycloplegic drug, patients with syndromes that interfere with the eye, and those who did not agree or sign the written informed consent, were excluded.
Descriptive statistics were used to characterize data from measurements of visual acuity and static refraction for both eyes. On the other hand, corneal keratometry, biometry and lens power calculations were registered only for the right eyes. For categorical variables, the chi-square test was performed together with the crude prevalence ratios and adjusted by the Poisson model with robust variance. All tests were considered significant when p<0.05 (5%).
3. RESULTS
Of the one hundred and forty-one university students who attended the invitation to participate in the study, one hundred and twenty-eight were eligible. Among the excluded patients, 2 were older than the selected population, 1 for keratoconus, 2 for strabismus and amblyopia and 7 for high astigmatism. The mean age was 21.28 ± 2.18 years. Forty-four (34.4%) participants were males and 84 (65.6%) were females. Regarding refractive errors, 18 (14.1%) were hyperopic, 41 (32%) were emmetropic, 61 (47.7%) were myopic and 8 (6.3) were high myopic. The mean and standard deviation of the SE, the AL, the keratometries (K1 and K2) and the powers of the lens of the total and in each refractive error were described in Table 1.
The distribution of refractive errors is found in Fig. (1). In relation to the family history of myopia, the groups were distributed as shown in Fig. (2). The average hours per week spent outdoors were 5.82 +/- 5.80 hs. and 2.18 +/- 2.37 hs. at sports activities at night. The distribution of AL correlated to SE was found in Fig. (3), where an AL increase of 1 mm corresponded with a SE change of 3.4 D. There was a positive correlation between refractive error and AL (R-Square 0.49, p=0.001).
Table 1. Mean and SD of principal linear variables
| Parameter | Mean | Standard deviation |
|---|---|---|
| Age | 21.28 | 2.18 |
| Age of the first prescription | 9.70 | 7.64 |
| Uncorrected visual acuity OD | 0.37 | 0.29 |
| Uncorrected visual acuity OS | 0.42 | 0.29 |
| Spherical equivalent OD | -1.27 | 2.21 |
| Spherical equivalent OS | -1.32 | 2.19 |
| Keratometry - K1 | 42.90 | 1.25 |
| Keratometry - K2 | 43.94 | 1.37 |
| Anterior chamber depth | 3.80 | 0.25 |
| Lens thickness | 3.48 | 0.20 |
| Axial length | 24.17 | 1.07 |
| Axial length emmetropic | 23.67 | 0.70 |
| Axial length myopic | 24.53 | 0.89 |
| Axial length high myopic | 26.15 | 1.07 |
| Axial length hyperopic | 23.21 | 0.57 |
| Lens power | 22.62 | 1.73 |
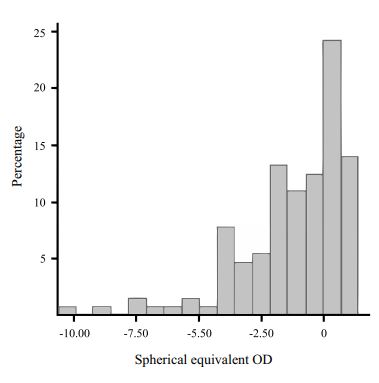 |
Fig. (1). Distribution of refractive errors. |
4. DISCUSSION
The myopia prevalence in the general Brazilian population reported for many years ago was 13%, which was quite low compared to values found in our study, of 47.7% myopic [2, 6]. A study in 2002 from the Brazilian northeast population, considering the age group 16 years or more, found a myopia prevalence of 15.81% [6]. It is important to consider that the cut point for myopia was based on spherical power and not on spherical equivalent, the most accepted nowadays. Currently, it was found that the children population from a rural northeast city of Brazil had around 20% of myopia (the mean age was 10.6-year only) [19]. Likewise, a higher prevalence of myopia among adults in the northeast Brazilian population will be expected, as it has been found for other populations [3, 6].
The adult population of the City of São Paulo had a mean AL 23.19 and 22.73 mm, for males and females, respectively. This was below the mean of AL 24.17 ± 1.07 mm, found in this study [20]. Myopic university students had a mean higher AL (24.53 ± 0.89 mm) in relation to emmetropes (23.67 ± 0.70 mm) and hyperopes (23.21 ± 0.57 mm). The results showed positive correlation between SE and AL, which was already reported in several studies [21-24].
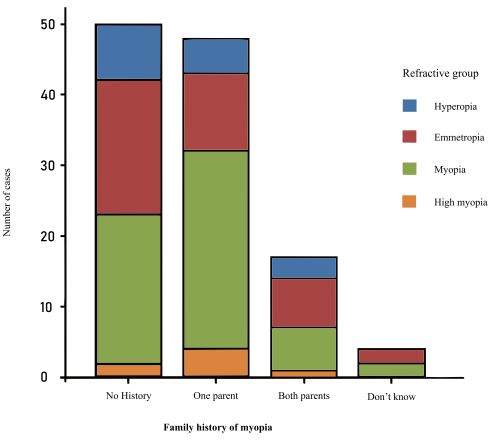 |
Fig. (2). Family history of myopia and refractive group. |
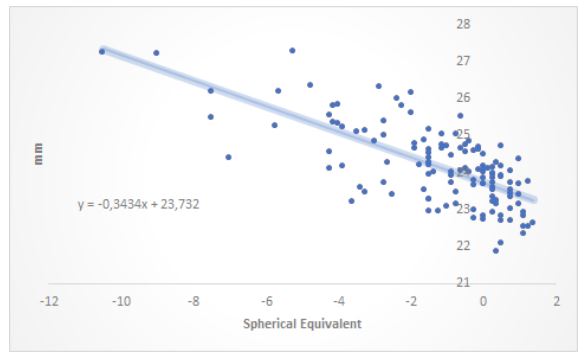 |
Fig. (3). Distribution of axial length and spherical equivalent. |
The choice of threshold point for high myopia was crucial in the present study. The World Health Organization – WHO - defines high myopia as <-5 D, however, currently, the International Myopia Institute - IMI – considers it to be <-6 D [8, 25]. If this study considered just < -6 D, we would had let 3 participants out of data from high myopia, but two of them already met an AL higher than 26 mm (26.19 and 27.28 mm), which is the main risk factor for the development of vision-threatening linked complications [26].
The family history of myopia was an often-associated factor, it was present in 62.50% of myopic university students. This high prevalence of family history in a sample with about 50% of young myopic subjects in a disease that is mainly environmentally driven speaks about the fact that family history is probably the history of shared genes and environments between parents and offspring [27].
Nevertheless, before arguing about the environment regarded the results of this study, we must consider the effects of the pandemic of Covid-19 over the lifestyle of humanity, who have spent a lot of time indoors for the last two years, with few hours of outdoors activities [28].
It needs to be noted, however, that the study has some limitations. First, the studied sample was based on the choice of six semesters at one medical university, representing a small number of people. There could also be a myopic bias in our invitation to students because, associated with this study, there was a longitudinal study about myopia.
CONCLUSION
The prevalence of myopia in this population was approximately three times higher than those reported for other samples of the Brazilian population. Studies in other cities and larger numbers of participants could help to find the real prevalence in medical university students from Brazil similarly to this study.
LIST OF ABBREVIATIONS
| AL | = Axial length. |
| SE | = Spherical equivalent. |
| UNIVAG-MT | = Centro Universitário de Várzea Grande, Mato Grosso, Brazil. |
| CAAE | = Certificado de apresentação para apreciação ética. |
| K | = Keratometry. |
| SD | = Standard deviation. |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was registered in the Plataforma Brasil Program – CAAE: 40738620.1.0000.5692.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
All participating students were oriented about the study, and those who agreed to participate signed written informed consent.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.







