All published articles of this journal are available on ScienceDirect.
Contact Lens as Drug Delivery System for Glaucoma Treatment: A Review
Abstract
Background and Objective:
Glaucoma is one of the leading causes of irreversible blindness globally and directly impacts optic nerve-altering vision. The condition has been linked to increased intraocular pressure (IOP). The objective of this review was to search how well different drug solutions containing gold nanoparticles (GNPs) work in treating glaucoma, with a focus on using contact lenses instead of regular eye drops.
Materials and Methods:
The methodology was structured to review different literature on ocular drugs used in contact lenses to investigate and determine their impact on intraocular pressure (IOP). Some of the intraocular drugs covered in the methodology include timolol, bimatoprost, pilocarpine, etc. The review focused on using gold nanoparticles (GNPs) infused with the solution in contact lenses for timolol.
Results:
The review found that timolol helps reduce intraocular pressure for the first two hours, but then the effect wears off. Moreover, gold nanoparticles infused with timolol solution on contact lenses improved IOP. GNPs in lenses increased the accumulation of timolol in ciliary muscles.
Conclusion:
Contact lenses with saturated drug solutions and GNPs have better bioavailability and release durations. Given its prolonged drug release time and bioavailability, the timolol solution relieves intraocular pressure better than other solutions. GNP-infused contact lenses with drug solutions have been found to treat glaucoma better than eye drops.
1. INTRODUCTION
The most common cause of irreversible blindness in the developed world is glaucoma, a group of optic neuropathies characterized by the progressive degeneration of retinal ganglion cells (RGCs) [1]. The typical symptoms include gradual loss of peripheral (side) vision, which is followed by central visual loss if left untreated. Glaucoma can proceed to total blindness if neglected. Only half of the carriers are aware of the disease since the damage to the eyes is gradual and painless, and typically permanent impairment occurs decades before diagnosis [2].
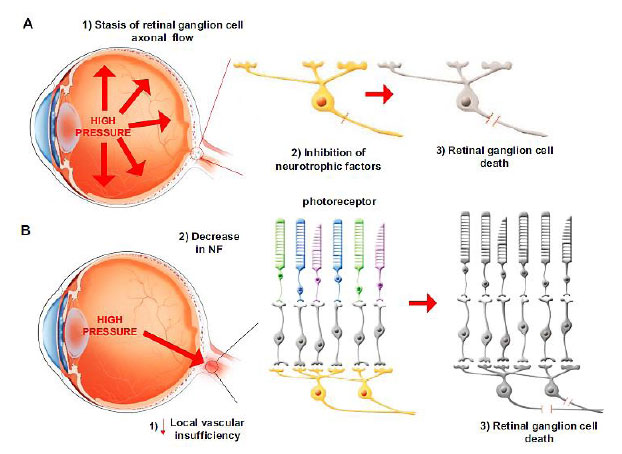
Glaucoma is typically diagnosed by abnormal intraocular pressure (IOP) regulation and pathological mechanosensitivity of ocular cells, but the relationship between mechanical forces and the disease is still unclear and of great interest to researchers, clinicians, and business leaders. While it is not always the case, an increase in intraocular pressure (IOP) is primarily believed to be the primary cause of this illness. There are different clinical kinds of glaucoma, but all have optic nerve degeneration. The optic nerve is destroyed by a process known as RGC apoptosis [1]. There are two main ways in which this apoptosis occurs. RGCs are damaged by mechanical damage, which is caused by higher IOP. High IOP stops the flow of RGC axons at the lamina cribrosa in the optic disc, which blocks neurotrophic proteins (NPs). The other cause is a lack of blood flow to the head of the optic nerve. This ischemic damage can cause the levels of NPs in the head of the optic nerve to drop, which kills RGCs (Fig. 1A and 1B).
1.1. Pharmacological Treatment
A reduction in intraocular pressure (IOP) is the only effective treatment for the management and prevention of glaucoma [3]. Drugs may affect intraocular pressure (IOP) by lowering the amount of aqueous humor produced by the ciliary processes, increasing the filtration rate of aqueous humor through the trabecular meshwork or uveoscleral pathways, or by the combination of both.
More than 90% of ophthalmic formulations available on the market are in the form of eye drops or ointments, indicating that topical administration is the preferred means of administration. According to a recent survey, the global ocular drug technology market was valued at $29.2 billion in 2016, and it is predicted to grow to $42.7 billion by 2023 [4]. Intraocular pressure (IOP) is a key risk factor for the disease, and eye drops are the first-line treatment for lowering it when it becomes elevated.
Eye drop solution is one of the most common treatments in eye care. It is subsequently washed away by tear drainage or lacrimation, which results in low bioavailability [5]. Although the eye drops reduce IOP, there are side effects, such as blurred vision, tachycardia, or an irregular heartbeat [4].
1.2. Contact Lenses as a Drug Delivery System
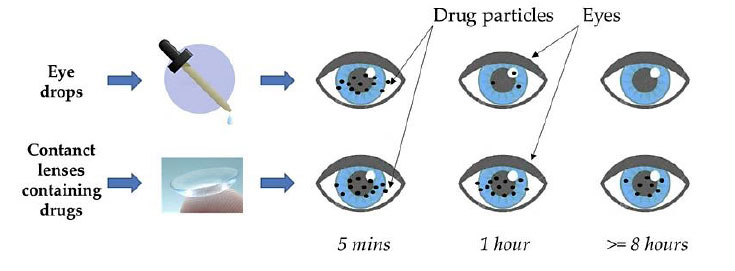
Systematic reviews focus on analyzing and discussing the in vivo efficacy of the contact lens (CL) as a drug delivery mechanism substitute for eye drops for glaucoma treatment. Due to increased residence time, contact lenses increase bioavailability [6]. The review states that, to date, only a nanoparticle-loaded CL or CL with implants can deliver medication for up to a week. Glaucoma therapy is more successful due to its more extended bioavailability (Fig. 2).
Contact lenses are custom-made polymer devices to fit the cornea to correct vision impairment. They are available in two types: hard and soft. As shown by these results, hydrogel contact lenses seem to be an effective method of delivering drugs to the eye [7]. Because of the contact lens's closeness to the cornea, the drug's bioavailability is more significant than other topically administered noninvasive ophthalmic treatments, like drops or ointments.
In 2022, 140 million people worldwide have been reported to wear contact lenses, and this figure is projected to grow in the coming years. When used as a drug delivery system, the CL has little effect on the everyday activities of these people. Furthermore, the CL could give excellent sustained drug delivery since it can release the drug for a few days after being inserted [8].
1.3. Drug Loading Techniques
Compared to traditional eye drop therapy, drug-eluting contact lenses perform better. New material development has provided contact lens wearers with several features, such as extended wear, high oxygen permeability, low mechanical modulus, and surface lubricity [9]. Several approaches have been investigated to improve the drug loading capacity and duration of drug release from commercial soft contact lenses (Fig. 3).
In recent decades, many lens-based drug delivery techniques have shown significant improvements in terms of bioavailability and drug residence time. Soaking diffusion barrier insertion, ligands, molecular imprinting, drug-loaded nanoparticles, and surface coating with colloidal nanoparticles or ligands via multilayer film deposition are some of the methods used to modify the surface of soft contact lenses (SCLs) [10] (Fig. 4).
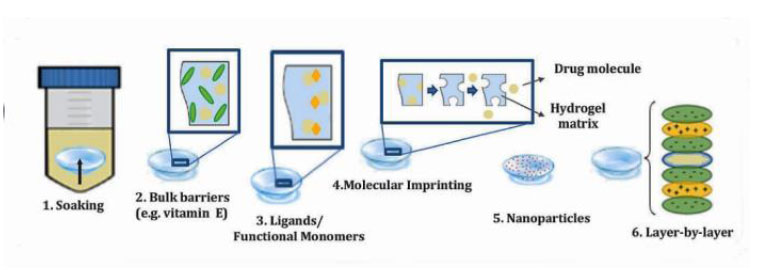
In addition to boosting drug retention on the cornea's surface, a drug-loaded contact lens may lower the amount of drug administered and the risk of unwanted effects while simultaneously increasing the bioavailability of the drug administered. In this approach, contact lenses may assist patients in adhering more strictly to their treatment regimen, eventually leading to better treatment outcomes.
It is being investigated if new and novel methods may be used to boost the loading capacity and release time of commercial soft contact lenses.
According to Peral et al. (2020), nanoparticle treatment may be more effective than the administration of drugs via contact lenses [11].
Maulvi and colleagues developed a hydrogel-encapsulated drug delivery system, which they then integrated into an ethylcellulose nanoparticle ring to administer the drug [12]. In glaucomatous rabbits, CL containing timolol was evaluated, and the results were found to be comparable to timolol eye drops. The IOP in the eye drop group had dropped by 4.4 mmHg after just two hours and had returned to baseline after 12 hours.
1.3.1. Soaking Method
The lens thickness, molecular drug weight, hydrophobicity, and soaking conditions, including time, concentration, and temperature, play a role in traditional soaking drug loading (Fig. 4, step 1). Patients may use commercial contact lenses, including hydroxyethyl methacrylate (HEMA), which is a significant benefit [13].
The soaking method is frequently used to insert drugs into contact lenses. The use of timolol-soaked contact lenses was first reported by Li et al. (2005), who discovered that they could provide timolol more effectively for a short period but could not be utilized for long-term ocular drug delivery [14].
Hiratani et al. (2005) produced timolol-loaded contacts via molecular imprinting. Imprinted lenses increased AUC (area under the curve) and medication retention in tear fluid with lower dosages than eye drop solutions [15].
1.3.2. Bulk Barriers
The incorporation of vitamin E aggregates into soft contact lenses results in the blocking of ultraviolet radiation and the creation of a hydrophobic diffusion barrier that prevents the rapid diffusion of drugs, all without compromising the lens's capacity to properly allow oxygen and ions through or its ability to properly refract light. Loading with vitamin E was shown to be very successful in extending the duration of release of timolol, bimatoprost, pilocarpine, and acetazolamide from a few hours to several days [16].
When loaded with vitamin E, contact lenses (CLs) revealed a timolol release time that was extended to 7-9 days, which was compared to the drug release duration shown by CLs that did not contain vitamin E loading. It is necessary to conduct in vivo transport and toxicity investigations before determining whether or not extended wear contact lenses serve this goal beneficially [17].
1.3.3. Ligands/Functional Monomers
Hydrogen bonds, electrostatic interactions, and host-guest interactions are all types of weak interactions between drugs and polymer ligands. Ions in a solution can enable it to load and release drugs in a controlled way. Depending on the charge of their functional groups, these ligands store either anionic or cationic drugs through an ion-exchange reaction. Hydrogels with cationic functional groups are good for releasing anionic drugs, while hydrogels with anionic functional groups are good for releasing cationic drugs. But the sustained release of these ions-ligand hydrogels only lasts a few hours, so they cannot be used for long-term drug delivery [18].
Hydrogels containing functional monomers that interact with the drug may have a longer release time. Increased drug interaction with these monomers retards the drug's diffusion from the hydrogel. Depending on the combination of the hydrophobic and hydrophilic components, the extended wear contact lens provided a prolonged release of timolol from 2 weeks to 3 months [19].
1.3.4. Molecular Imprinting
Molecular imprinting creates a template based on a macromolecular memory within a flexible network with a better drug molecule affinity. Functional monomers should interact favorably with a certain drug, improving drug uptake and delivery. Imprinting increases partitioning depending on CL functional monomer percentage, temperature, pressure, drug-functional monomer ratio, initiator concentration, and crosslinking degree. Imprinted hydrogels comprised of hydroxyethyl methacrylate (HEMA) and small amounts of methacrylic acid (MAA) showed better timolol absorption (12 mg/g dry hydrogel) than non-imprinted gels and 8-10 hours of drug release [20].
In imprinting research, timolol is the drug that is utilized the majority of the time; however, hyaluronic acid, acetazolamide, ciprofloxacin, and prednisolone have also been studied [21].
1.3.5. Nanoparticles
Colloidal nanoparticles are submicron-sized particles that either encase the agent molecules or mix with them. Liposomes and colloidal polymeric nanoparticles are two common types. Because they are biocompatible, liposomes are good candidates for spreading or sticking to the surface of CLs. Nanoparticles are made to exhibit a strong attraction for the drug of interest. They can be mixed into the matrix or coated on the surface of the CL. Once the nanoparticle is put into the eye, the agent moves out of the CL matrix and toward the tear film. Timolol-loaded nanoparticles in HEMA-based CLs kept the drug stable when it was kept in the fridge, and the change in temperature caused the drug to be released when the CL was put in. Timolol was released from nanoparticle-loaded gels into phosphate-buffered saline (PBS) for 2 to 4 weeks at therapeutic levels, which is a good sign for long-term drug delivery [22].
1.3.6. Layer-by-Layer
Layer-by-layer platforms have been used to deliver drugs because they are easy to make and do not react adversely with mild aqueous at chamber temperature. By putting a layer of poly [lacticco-glycolic acid] (PLGA) between two layers of 100-micron-thick gel, the release of timolol was lengthened from two weeks to three months. A hydrogel with a layer of ciprofloxacin-loaded PLGA film in between two layers of pHEMA was able to release medicine slowly and stop bacteria from growing. The release of different ophthalmic drugs from a silicone-based hydrogel was tested by adding layers of chitosan and alginate. A double layer of this coating made sure that the diclofenac was slowly released for one week [23].
1.4. Nanomedicines for the Treatment of Glaucoma
The development of nanodelivery technologies for anti-glaucoma medications has made nanotechnology increasingly essential in treating glaucoma [24]. Eye medications, incisional therapy, and laser surgery have been extensively studied to slow the course of the disease and reduce intraocular pressure (IOP) [25]. Various challenges have hampered glaucoma treatment, but medical researchers and practitioners have developed promising ways to overcome these obstacles using nanotechnology. In the near future, nanotechnology will have revolutionary effects on biology and medicine and may be utilized to research and manage many biological and medical processes. Applications for nanoparticles range from biosensors to image enhancers and delivery agents [26].
1.4.1. Gold Nanoparticles as a Drug Delivery System
Since its discovery, gold has been focused in scientific inquiry and for practical use. Nanoparticles of gold are an attractive study material for various reasons: they are stable, nontoxic, easy to create, and have interesting properties, such as the capacity to form multiple assemblies and the quantum size effect [27].
Gold nanoparticles (GNPs) have shown great potential in ophthalmology for diagnostic and therapeutic applications [28]. GNPs can be utilized in the administration of drugs because of their high stability. According to Masse et al. (2019), their ultrastability increases their efficiency while preventing accumulation in the body. Because of this, glaucoma sufferers may benefit from GNPs [29].
Nanotechnology advancements are paving the way for the better treatment of eye disorders in the future. Reduced eye irritation and higher drug bioavailability are two benefits achieved. Payloads are transported to the eye's posterior and anterior chambers by nanosystems involving gold nanotechnology. Several common colloidal nanosystems include micelles, liposomal hydrogels, and cyclodextrins [30]. Therefore, nanosystems technology has significantly improved the treatment of anterior segment diseases.
In the summary article by Masse et al. (2018) reviewing gold nanoparticles in ophthalmology, it was shown that GNPs might be used to transmit genes or drugs, treat oxidative stress, and measure intracorneal pressure [31].
According to a study by Occhiutto et al. (2019), nanotechnology may be used to treat eye conditions [32]. According to Sonntag et al. (2021), nanoparticles may transport drugs to the eye's outflow tissues, where the disease occurs [4].
However, according to Sani et al. (2021), research by medical experts reveals that GNPs may pose a health risk. The chemical and physical characteristics of nanoparticles significantly impact toxicity and its extent [33]. As the patient compliance is reduced due to irritating eyes when eye drops are given [1], contact lens treatment is necessary (Table 1).
2. Ocular Drugs Loaded with Contact Lenses
The published laboratory research on glaucoma treatment has proven that the use of contact lenses saturated with drug solution and gold nanoparticles leads to more bioavailability, release, and a longer duration of action for the drug than treatments using conventional eye drops due to their biodegradable nature. Patients with high intraocular pressure who also have glaucoma can be treated with timolol, bimatoprost, pilocarpine, acetazolamide, latanoprost, and dorzolamide [37].
2.1. Timolol
Maulvi et al. (2019) investigated the effect of timolol on the treatment of glaucoma through a soaking technique. The use of timolol in a conventional solution showed high burst release and low drug loading. GNP was infused with the timolol solution in one technique, while GNP was incorporated directly into the contact lenses in the second technique. In vitro results were similar, but in vivo GNP infused with timolol lowered intraocular pressure by 2-4 mmHg (72h). The drug loading time increased in both the studies. Through this technique, drug limitations, such as the high burst rate, were controlled with the use of GNP without altering the lens properties [13]. Later, the experiment was used to study gold nanoparticles and their release kinetics from contact lenses [38].
Peng et al. (2012) performed in vivo and in vitro studies on beagle dogs to compare the effects of contact lenses infused with timolol with eye drops [39]. The results of timolol were observed by having the dogs wear contact lenses for an extended duration [38]. The efficacy of timolol for open-angle glaucoma was then compared with eye drops. The lens was used for 24 hours to observe the results. The in vivo studies showed that the intraocular pressure decreased from baseline by using timolol-infused contact lenses [13]. The reduction in intraocular pressure was 19-29% compared to that of the eye drops.
| Factor | GNPs | Contact Lens |
|---|---|---|
| Drug release | GNP triggers and controls the release of the drug into the eye [34]. | The drug release is sustained throughout the active period of the medicated lens [9]. |
| Bioavailability | It has a low bioavailability since drop dispensers have a higher volume capacity than the conjunctival sac leading to a lot of the liquid draining out [4]. | The bioavailability is increased by 50% due to the direct contact of the lens with the cornea and the prolonged drug release [6]. |
| Ocular discomfort | Slight discomfort when administering the eye drops [1]. | Discomfort is suppressed due to the current use of soft contact lenses [35]. |
| Compliance | Poor patient compliance is due to frequent administration cycles [1]. | Contact lenses are safe and comfortable, which leads to perfect compliance [35]. |
| Irreversibility of glaucoma | Glaucoma-induced vision loss is not reversible under GNP treatment [25]. | Treatment with contact lenses prevents deterioration due to glaucoma because contact lenses can detect any changes in the IOP [36]. |
| Half-life | The half-life varies with different nanoparticles, but it ranges between 2-3 hours, requiring frequent drug administration during the day [26]. | A contact lens with timolol could function for as long as two weeks [10]. |
The results obtained through the existing literature showed that gold nanoparticles infused with a drug solution, such as timolol, showed greater bioavailability, lower burst rate, more extended drug release, and were more effective in lowering intraocular pressure compared to eye drops, which have poor bioavailability [38].
According to Choi and Kim (2018), nanoparticle-embedded CL showed an extended release of timolol constantly over one month [40]. However, according to a recent study by Zhai et al. (2021), drug-loaded nanoparticles in contact lenses can alter intrinsic contact lens properties, such as mechanical properties, optical transmittance, and oxygen penetrability due to nanoparticle distribution all through the contact lens structure [34].
2.1.1. Timolol Release from GNPs-Laden Contact Lenses In vitro
2.1.1.1. GNPs-CL immersed in timolol (2 mg/ml)
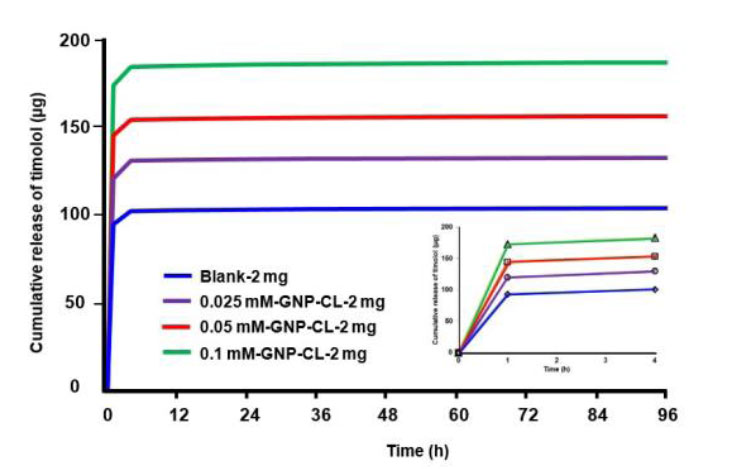
Fig. (5) shows the cumulative timolol release profiles (µg) from blank and GNPs-CL immersed in 2 mg/ml timolol solution. 0.025 mM-GNP-CL-2 mg, 0.05 mM-GNP-CL-2 mg, and 0.1 mM-GNP-CL-2 mg released 131 µg, 155 µg, and 185 µg, respectively, while blank-2 mg (without GNPs) released 103 µg (4 days) [13].
2.1.1.2. GNPs-CL immersed in timolol (4 mg/ml)
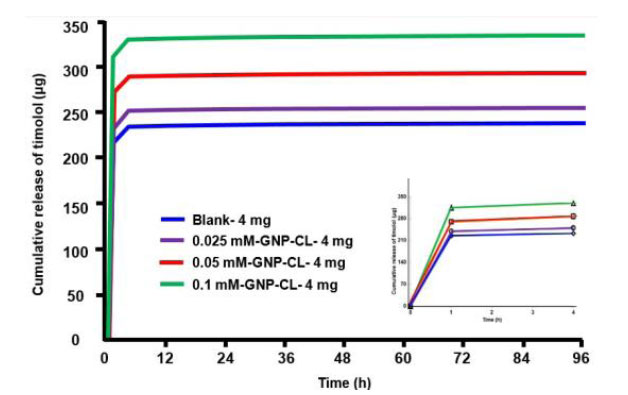
Fig. (6) shows the cumulative timolol release profiles (µg) from blank and GNPs-CL immersed in 4 mg/ml timolol solution. 0.025 mM-GNP-CL-4 mg, 0.05 mM-GNP-CL-4 mg, and 0.1 mM-GNP-CL-4 mg released 252 µg, 290 µg, and 333 µg, respectively, while blank-4 mg (without GNPs) released 237 µg. (4 days).
2.1.2. The Drug Release Study In vivo
After using GNPs-loaded contact lenses, the concentration of timolol (µg/gm) in ocular tissue increases by two-fold and four-fold in the iris-ciliary and conjunctival tissues.
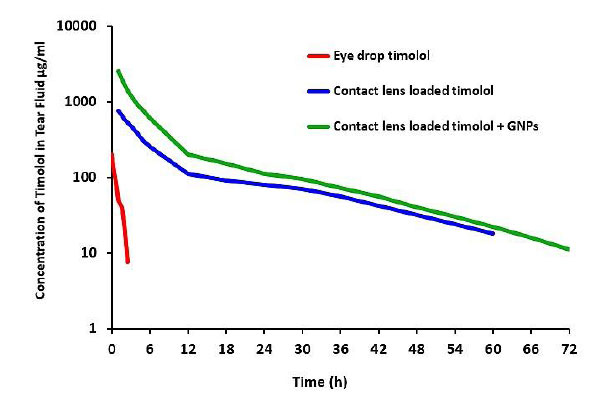
The concentration of timolol in tear fluid was measured after treatment with 0.025 mM-GNP-CL-4 mg (277 µg timolol loading), blank contact lenses-4 mg (253 µg timolol loading), and eye drop treatment (1 drop = 250 µg of timolol), as shown in Fig. (7); the details of concentration are described as follows:
- In the eye drop, the maximal timolol concentration was 133 µg/ml in rabbit tear fluid, which was reached at 5 min, followed by an exponential fall with no drug detectable after 2 h.
- In contact lens loaded with timolol, the maximum concentration of timolol was 581 µg/ml after 1 hour.
- In contact lens loaded with timolol and GNPs, the maximum concentration of timolol was 1685 µg/ml after 1 hour. The initial release was quick (1 hour), followed by a 72-hour steady release.
2.1.3. Study of Pharmacodynamics In vivo
The normotensive IOP of GNPs-loaded contact lenses, blank lenses-4 mg (timolol loading), and eye drops was 14.5 mmHg. Fig. (8) describes the details of high intraocular pressure as follows:
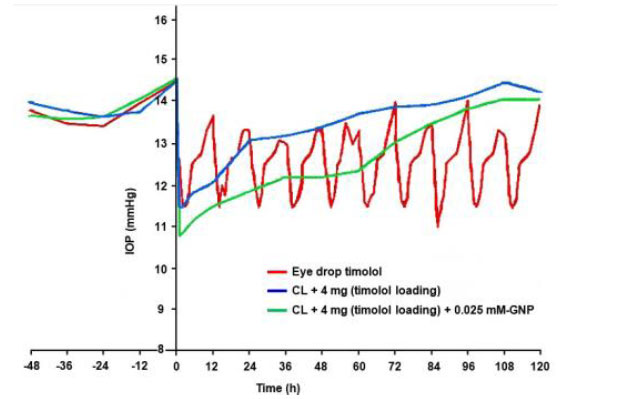
- After 3 hours of eye drops administration (multiple installations every 12 hours), the highest IOP was lowered by 3 mmHg (from baseline) and returned to normal after 12 hours, with a 2 mmHg average reduction.
- After one hour of CL-4 mg (timolol loading), the highest IOP drop was 3 mmHg (from baseline), returning to normotension after 72 hours with a 2 mmHg average reduction.
- After one hour of GNP-loaded contact lenses, the highest IOP drop was 4 mmHg (from baseline), returning to normotension after 72 hours with a 2 mmHg average reduction.
2.1.4. Distribution of Gold NPs in the Outflow Tissue
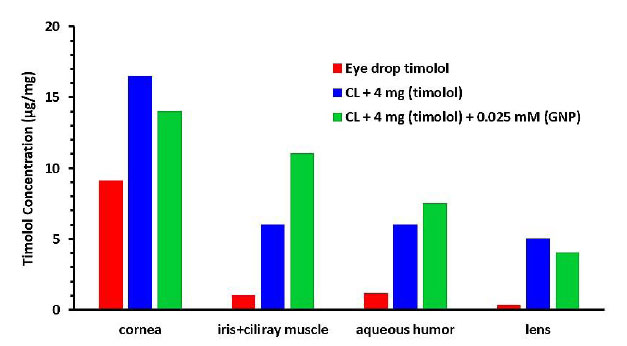
After 5 hours of contact lens wear, the concentrations of timolol (μg/mg) in the cornea, iris-ciliary muscle, aqueous humor, and lens in rabbit eyes are shown in Fig. (9). The amount of timolol in the ocular tissues was greater in the eye drop solution group.
GNP-loaded contact lenses showed no significant increase in timolol concentration in most ocular tissues except the iris-ciliary muscle and aqueous humor compared to blank-4 mg contact lenses.
The extended IOP decrease in GNPs lenses may be due to timolol accumulation in the ciliary muscle, which contains most β-receptors [36]. GNPs in the contact lenses enhanced drug permeability and accumulation in the ciliary muscle tissues.
2.2. Bimatoprost
Li et al. (2021) performed a study to understand the effect of bimatoprost on intraocular pressure by testing it with a conventional solution and then using a contact lens infused with gold nanoparticles [41]. First, bimatoprost showed a high burst release, poor drug uptake, and critical lens properties compared to the conventional solution. Later, the effects of gold nanoparticles and their release kinetics were investigated. The nanoparticles of 21.1 nm were included in the contact lenses [40, 41].
The optical transmittance and oxygen permeability of the drug improved after soaking it with gold nanoparticles. The in vitro studies showed a significant improvement as the drug's sustained release time increased from 24 to 36 hours with a low burst rate. The gold nanoparticles helped in lowering protein adherence [38].
2.2.1. The Drug Release Study In vivo
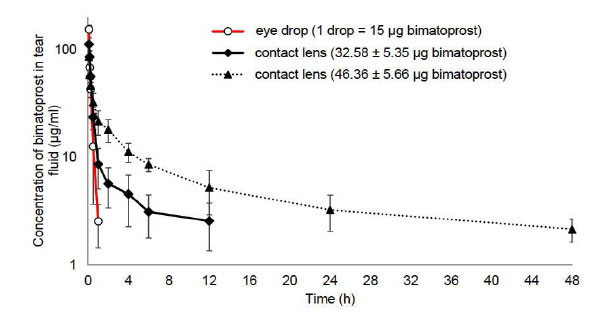
In vivo tear fluid analysis was performed using bimatoprost-loaded contact lenses (32.58 ± 5.35 μg), (46.36 ± 5.66 μg), and 0.03% w/v eye drop (1 drop = 50 μl = 15 μg bimatoprost). Bimatoprost's Cmax (5 minutes) was estimated to be 112.25, 85.69, and 154.69 μg/ml (Fig. 10), respectively. Bimatoprost eye drop solution demonstrated rapid drug concentration loss (up to 1 h), but contact lens-loaded bimatoprost retained the drug for 12 and 48 h, respectively [42].
2.3. Pilocarpine
Using GNP-loaded contact lenses to deliver the drug provides an effective ophthalmic sustained-release formulation of pilocarpine for treatment through improved bioavailability [8]. Studies show that the duration of the response has increased by 20% for the nanoparticle formulation. The nanoparticles significantly decreased the IOP without drug-induced side effects. By reducing the absorption rate, the irritation caused by pilocarpine was reduced [43].
2.3.1. In vitro Pilocarpine Release from Nanoparticles
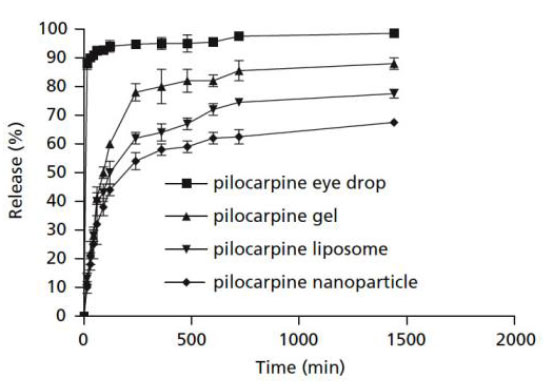
Pilocarpine in eye drops was quickly released, and within 4 hours, more than 90% of the loaded pilocarpine had been released and reached a plateau (Fig. 11). Pilocarpine loaded into nanoparticles, liposomes, and gel showed a burst-release at first, followed by a steady release for 24 hours. Nanoparticles were found to be the best of the four ways to keep pilocarpine in the body for the longest amount of time [44].
2.4. Acetazolamide
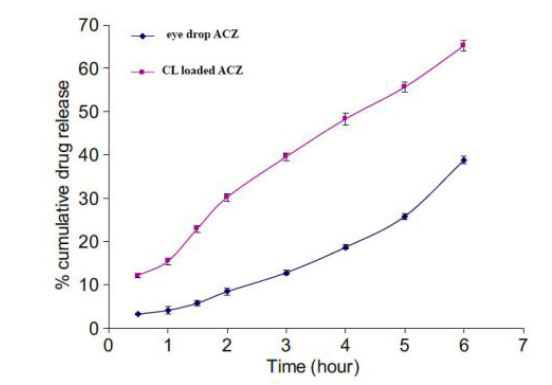
Acetazolamide (ACZ) drug-loaded GNPs contact lenses indicate efficient drug delivery and fewer drug-induced side effects [45]. A study showed ACZ-loaded nanoparticles to display better permeability and flow across the cornea than conventional drug delivery (Fig. 12) [46]. The study further demonstrated a reduction in IOP and improved ocular tolerability. The nanoparticles did not harm the eye, as no adverse effects were reported. The ACZ drug-loaded nanosystem has many advantages as it enhances the solubility and bioavailability of drugs [40, 46, 47].
2.5. Latanoprost
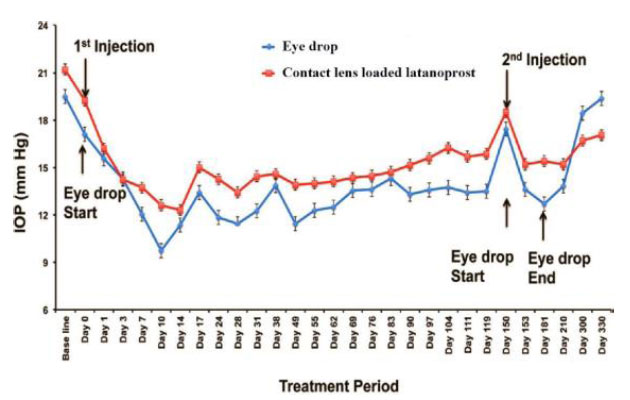
In the study by Alm (2014), latanoprost-loaded GNP was administered, and the drug released from GNP was sustained over 14 days. The nanoparticles induced a significant ocular hypotensive effect. However, the efficacy was found to be limited and the adverse effects have been reported to lead to poor adherence and treatment persistence [48]. No ocular inflammation was seen in the NP-treated group [8]. IOP may be reduced for up to 120 days after a single injection of contact lens loaded with latanoprost, and the results were found to be comparable to those using daily topical eye drops. After that, a second injection was given, and the intraocular pressure continued to fall throughout the duration of the subsequent 180 days (Fig. 13) [25].
2.6. Dorzolamide
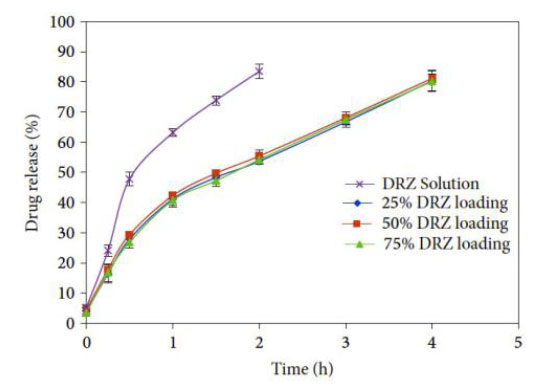
Dorzolamide, often known as DRZ, is a drug that is frequently utilized in the treatment of glaucoma. The inhibition of carbonic anhydrase leads to a reduction in the production of aqueous humor and an overall decrease in intraocular pressure. Nikouei et al. (2013) and Juliana et al. (2019) discussed that carbonic anhydrase inhibitors, such as dorzolamide, are often used in eye drop solutions to treat glaucoma. Dorzolamide lowers IOP by inhibiting the ciliary body's generation of aqueous humor [25, 48]. This reduces the amount of fluid in the eye, lowering its pressure. Its effect on the eye includes eye irritation. GNPs encapsulate, conjugate, or adsorb the drug in nanoparticles, which improves the structural and functional activities of the cornea. The release is sustained up to 8 h; contact lens-loaded NPs also follow a biphasic release pattern with burst release of 30 min duration, releasing 30% to 35% of the drug and providing the sustained release period of 4h (Fig. 14) [32].
2.6.1. The Drug Release Study In vivo
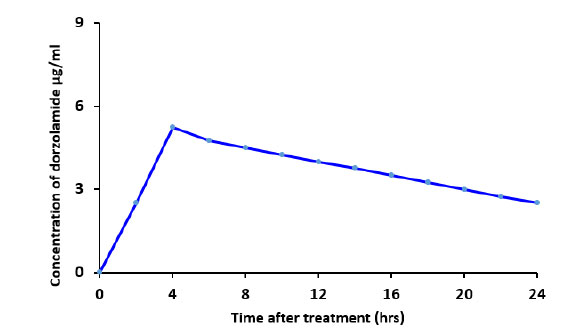
After topical administration of the aqueous dorzolamide eye drop suspension to rabbits, no macroscopic symptoms of irritation or redness were seen. The concentration of dorzolamide in aqueous humor following topical application of the 3% (w/v) dorzolamide eye drop suspension is shown in Fig. (15). The highest concentration (Cmax) of the aqueous 3% (w/v) dorzolamide eye drop suspension comprising dorzolamide with nanoparticles and in solution was 5.4 µg/ml at 4 hours after topical application (tmax = 4 h) (Tables 2 and 3) [50].
After collecting the research on these different drugs and comparing them, it was found that timolol drug-eluting contact lenses release medicine at a higher therapeutic concentration than typical eye drops throughout the day, as shown in Fig. (16). Timolol CL improves therapeutic efficacy by prolonging drug release time, increasing drug bioavailability, and reducing side effects.
| Ocular Drug | Condition | Results |
|---|---|---|
| Timolol | In vivo | In the GNP-laden CLs group, IOP dropped after 1 h and increased after 72 h. In the eye drop group, IOP increased after 3 h and returned to baseline after 12 h [13]. |
| Bimatoprost | In vitro | Increased time release (>10 days) but reduced light transmission [51]. |
| Pilocarpine | In vivo | Ocular irritation, induced myopia, and decreased vision due to ciliary spasm [11]. |
| Acetazolamide | In vitro | Increased time release (24 days), ocular irritation, and dry eyes [11]. |
| Latanoprost | In vivo | Conjunctival hyperemia, change of iris color, uveitis, macular edema, and keratitis [52]. |
| Dorzolamide | In vivo | Increased hypotensive effects for 8 days [49]. |
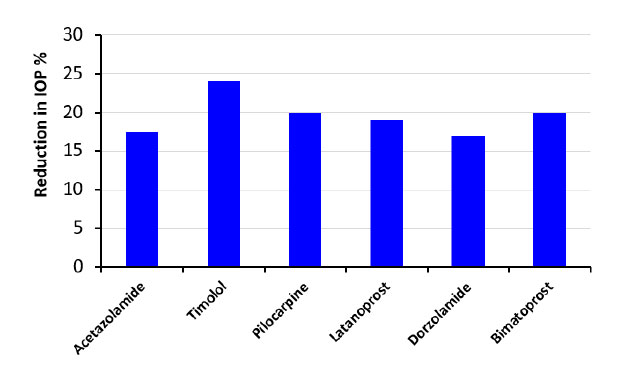
| Ocular Drug | Drug Concentration | The Period in the Eye | Reduction in IOP |
|---|---|---|---|
| Timolol | 2-4 mg/ml | The onset of the intra-ocular pressure reduction can be realized in 30 minutes after a single dose is administered. The maximum effects usually occur in 1-72 hours [13]. | 19-29% |
| Bimatoprost | 3 mg/ml | It may take at least four weeks before the results are realized [41]. | 22% |
| Pilocarpine | 10 mg/ml | The pilocarpine-soaked contact lens was found to be as effective at lowering IOP as intensive, high-concentration (40 mg/ml) pilocarpine drop therapy [53]. | 20% |
| Acetazolamide | 0.1 mg/ml | ACZ-loaded NPs displayed better permeability and flow across corneal tissue than the drug suspension, and ocular insert demonstrated substantial IOP lowering and improved ocular tolerability when compared to ACZ suspension [32]. | 15-20% |
| Latanoprost | 50 mg/ml | The maximum aqueous humour concentrations were much higher with contact lens treatment than with drop instillation, and the concentrations stayed relatively constant between 3 and 28 days [54]. | 18-20% |
| Dorzolamide | 20 mg/ml | IOP measurements after a single dosage (after 30 min of drug administration and then every 1 h over a period of 8 h) [32]. | 15-19% |
According to the research, incorporating GNPs into contact lenses improves drug uptake, allowing for sustained release and reducing IOP. GNPs were added to contact lenses (GNPs-CL) to investigate how gold nanoparticles affect contact lens loading. Although not involving the presence of GNPs swelling or transmittance in the CL, timolol concentrations were 2 mg/ml and 4 mg/ml in contact lens solutions.
CONCLUSION
Glaucoma can permanently destroy an eye's optic nerve if left untreated. Medicated eye drops are one of the best ways to treat glaucoma, although it has been shown that they are not suited for all glaucoma patients. As a result of over a decade of research, the scientific community has found a method of using contact lenses to cure glaucoma. Although eye drops contain a higher dosage of drugs, contact lenses have proven to be more effective. Timolol-contact lenses with GNPs have been shown to increase the drug's efficacy and reduce IOP within 24 hours of introduction.
Maulvi et al. (2019) discovered that the longer it takes for the ocular drug to operate, the longer it is essential to use contact lenses. This is due to the longer duration of timolol release and the higher permeability of these contact lenses, which contributes to the extended period of contact lens usage. Timolol-loaded CL concentrations of 4 mg/ml help in drug absorption and release. The pharmacodynamic analysis of the soaked CL and eye drop solution showed a 2-4 mmHg decrease in IOP.
The review has found that GNPs increase in concentration or soaking solution increases timolol’s cumulative release. GNPs in CLs improve timolol’s uptake, which aids in glaucoma treatment. Timolol has a 4.3 mg/ml solubility near the medication's soaking strength, explaining why timolol has high uptake. The research also indicates that timolol is a low-molecular-weight drug that sticks to GNPs' surfaces and manifests inside the CLs matrix, increasing the aqueous humor's infiltration rate. The process lowers the IOP by about 2 to 4 mmHg, especially when timolol is GNP-infused.
To conclude, GNP-loaded contact lenses increase timolol release kinetics while simultaneously decreasing intraocular pressure (IOP). The findings also suggest that CLs increase timolol's bioavailability, reducing IOP from baseline. Other eye drops' efficacy has been found to lag by about 29% compared to timolol. The treatment method (timolol plus CLs infused with GNPs) lengthens the precorneal residence duration. The amount of drug absorbed into the tissue five hours after the delivery was found to be more significant than the amount absorbed 48 hours later.
LIST OF ABBREVIATIONS
| IOP | = Intraocular Pressure |
| RGCs | = Retinal Ganglion Cells |
| NPs | = Neurotrophic Proteins |
| CL | = Contact Lens |
| HEMA | = Hydroxyethyl Methacrylate |
| MAA | = Methacrylic Acid |
| ACZ | = Acetazolamide |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


