All published articles of this journal are available on ScienceDirect.
Transcutaneous Radiofrequency-mediated Meibomian Gland Expression is an Effective Treatment for Dry Eye: A Prospective Cohort Trial
Abstract
Purpose:
Meibomian gland dysfunction disrupts tear film homeostasis and results in dry eye syndrome. The objective of this study is to determine whether transcutaneous radiofrequency (RF)-assisted meibomian gland expression, using the Envision platform and Forma-I handpiece is an effective treatment for dry eye syndrome.
Methods:
A multicenter prospective cohort study of patients with dry eye undergoing RF-assisted meibomian gland expression was completed from October 2019 to June 2022. The study was designed with multiple primary endpoints. These were defined as the change in Standard Patient Evaluation of Eye Dryness (SPEED) score, changes in Ocular Surface Disease Index (OSDI), Tear Breakup Time (TBUT), Corneal Fluorescence Score (CFS), and Meibomian Gland Score (MGS) at 1, 3, and 6 months after treatment. Secondary endpoints were measurements of patients' subjective improvement and subjective satisfaction with the treatment.
Results:
A total number of 47 patients were enrolled in the study at three separate institutions by three ophthalmologists (oculoplastics, refractive, and general). However, not all subjects had complete data on all observations at all time points following treatment. When a data point was missing, the entire patient’s outcome for that data set was excluded from the analysis. There was a significant improvement in SPEED score from baseline, 15.7 vs 11.4 at 1 month, 9.1 at 3 months, and 9.6 at 6 months (p<0.05). There was also a statistically significant improvement noted for OSDI at all time points measured, 34.5 at baseline vs 25.2 at 1 month, 21.2 at 3 months, and 23.6 at 6 months (p<0.5). CFS was significantly reduced in each eye at all time points following treatment as well, with 80% of eyes responding. TBUT similarly improved after treatment in each eye with an average of 6.3s at 1 month, 7.1s at 3 months, and 7.1s after treatment at 6 months vs 2.8s at baseline. The MGS also showed marked improvement across all time points, 5.6 at baseline vs 19.9 at 1 month, 24.7 at 3 months, and 22.9 at 6 months. Patients noted subjective improvement, with a lack of pain and discomfort from the treatment.
Conclusion:
This pilot study demonstrates that RF treatment with the Forma-I handpiece along with meibomian gland expression is an effective means to reduce the signs and symptoms of dry eye disease. The data support the conclusion that the treatment is safe and effective, lasting at least 6 months in most patients.
1. INTRODUCTION
Meibomian gland (MG) dysfunction secondary to obstruction is considered one of the leading causes of dry eye [1]. Epithelial hyperkeratinization results in terminal duct obstruction, meibum stasis, duct dilation, and inflammation [2]. The two major risk factors are aging and environmental stress. With aging, reduced cell turnover promotes increased inflammatory infiltration, and decreased lipid production results in stasis. Environmental stresses such as low humidity promote gland obstruction, while a higher protein/lipid ratio increases the viscosity of meibum leading to stasis. Clinically, slit lamp inspection demonstrates meibomian orifice plugging, hyperemia, telangiectasias, and changes in orifice position. With mechanical pressure applied to the eyelid the rate of expressibility and texture of the meibum can support a diagnosis. Ocular surface damage results from increased tear evaporation, decreased lubrication and a preponderance of proinflammatory mediators. While the severity of MGD has been shown not to correlate with symptoms, many patients are debilitated with pain, photophobia, and blurry vision.
Conventional approaches to dry eye include lid massage, warm compresses, and manual expression. Massage and manual expression are limited by pain tolerance and the success of warm compresses is variable. Inconsistent results have inspired researchers to look for innovative ways to relieve meibomian gland obstruction. Recently, LipiFlow (Morrisville, NC) and Intense Pulse Light (IPL) have emerged as promising technologies that work to heat the lid margin and help restore gland flow.
Today, radiofrequency (RF) deliveries have become common to the provider’s armamentarium. These devices deliver the energy that differentially heats the tissue by the impedance of the electromagnetic current. The differential heating across the tissue results are consistent with Ohms Law (Energy= Current x Impedance x Time). RF devices have been used for aesthetic and non-aesthetic treatments and their popularity continues to grow.
This study aimed to investigate whether transcutaneous RF could be safely and effectively applied to eyelids to improve meibomian gland function and improve symptoms of dry eye. We hypothesized that RF-mediated eyelid heating using the Forma-I handpiece would provide a novel treatment for dry eye disease.
2. METHODS
2.1. Study Design
A multicenter prospective study (REF#7546-D0609175A), approved by Sterling Institutional Review Board) was performed from October 2019 to June 2022. There was no ethics committee approval. The inclusion criteria included; 1) age 18 -75; 2) Tear breakup time (TBUT) ≤10 seconds; 3) evidence of meibomian gland (MG) obstruction based on total MGS of ≤12 in lower eyelids for each eye; 4) subjective symptom score (using the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire) ≥10; 5) At least one meibomian gland opening with a visible plugging over the eyelid margin; 6) no ocular pathology requiring treatment other than eye lubricant and conventional eyelid hygiene within the last month and during the study; and 7) Ability to abstain from dry eye/MGD medications or any device treatments for the time between the treatment visit and the final study visit. Patients were excluded if they had any intraocular inflammation, previous ocular surgery, ocular trauma in the past 12 months, ocular infection, eyelid structural abnormality, systemic diseases that lead to dry eye disease, skin cancer, pregnancy, or lactation. Patients who wore contact lenses were not included in the study.
Patients were screened for the study using the SPEED questionnaire, OSDI, TBUT measurement, meibomian gland score (MGS), and corneal fluorescein staining (CFS) assessment. Patients who satisfied the selection criteria were informed about the study and provided written consent to participate. The ethical management of patients and data was performed in keeping with the Helsinki protocol.
2.2. Treatment Protocol
The Envision platform equipped with the Forma-I handpiece (InMode Ltd., Yokneam, Israel) was used to provide transcutaneous radiofrequency (RF) energy The Forma-I handpiece was specifically designed to treat periorbital skin as shown in Fig. (1). The distance between the two electrodes limits RF penetration depth to 2.5 mm. Ultrasound gel was used to aid in the conduction of energy into the tissue. Using this technique, the tissue was not ablated nor an open wound created. The handpiece monitored the temperature and controlled the RF energy to maintain the heat at predetermined levels in the range of 40 - 43°C. After cleaning the treatment area, approximately 2-3 mm of clear ultrasound gel was applied. Treatment parameters were set according to the subject's tolerance (Energy 5-20). Cut-off temperatures were set between 41.5° - 42°C, starting at 40 degrees, and gradually increased according to the subject's tolerance. Full contact of the handpiece with the skin with a slight pressure was maintained, with a hand flattening and stretching the treatment area as needed. After pressing the footswitch to initiate the RF energy the handpiece was moved from tragus to tragus back and forth with gentle pressure over the full treated zone to reach uniform skin heating. The movement was constant to avoid hot spots. The operator then retracted the brow and forehead skin to allow exposure and treatment of the upper eyelids. The treatment started at a low setting level to monitor the patient’s tolerance to the treatment parameters. The total treatment time was 12 minutes per side after which, a drop of topical anesthetic was placed into both eyes and therapeutic meibomian gland expression was performed at the slit lamp with Collins Expressor Forceps (Bruder). At one study site a protective eye shield utilizing a flexible handle was used for further ocular protection and to serve as an additional base for gland expression. With the shield over the anesthetized eye, the tip of the handpiece was able to treat along the eyelid margin for heating and then used to compress the outer lid and “ski” down the tarsal plate to express the oils while the lid was at maximal heat from the RF device. This technique had been described using a monopolar RF device compared to Lipiflow (Pelleve, an Ellman device no longer in production) [3]. Standardized outcome measurements were collected at baseline, 1 month, 3 months, and 6 months thereafter.
2.3. Outcome Measurements
2.3.1. Primary Endpoints
The Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire was used to assess patient symptoms. SPEED was assessed via a scale anchored by 0 - 4 (mild symptoms) and 8+ (severe symptoms). SPEED responders were defined as those who had a 4-point improvement from baseline to 6 months post-procedure. A subset of this group was defined as extreme responders or those that had an 8-point reduction from baseline to 6 months.
The Ocular Surface Disease Index (OSDI) questionnaire is a valid and reliable instrument for measuring dry eye disease severity (normal, mild to moderate, and severe) and the effect it has on visual function. The OSDI has twelve items designed to provide a rapid assessment of the symptoms of ocular irritation consistent with dry eye disease and their impact on vision. It is measured on a scale of 0 to 100, with higher scores representing greater disability. The index demonstrates sensitivity and specificity in distinguishing between normal patients and patients with dry eye disease. ODSI was assessed via a scale anchored by 0-12 (normal) and 33+ (severe disease). ODSI responders were defined as those who had a 10-point improvement from baseline to 6 months after the procedure.
Tear breakup time (TBUT) was assessed via a scale anchored by less than 5 seconds (dry eye) and greater than or equal to 10 seconds (normal). Both eyes were evaluated independently. Tear break-up time (TBUT) was then performed by placing one drop of fluorescein into the lower fornix of each of the patient's eyes. The eye was then examined under a slit lamp with low magnification (10x) and a broad beam with a cobalt blue filter covering the whole cornea. The patient was then asked to blink once and to keep their eyes open. A black spot indicating the dry area appeared a few seconds after each blink. The TBUT measured the time interval between the last blink and the appearance of the first randomly distributed dry spot. Taking two or more measurements and calculating their average was utilized to give greater accuracy.
Corneal fluorescein staining (CFS) was performed for each eye at baseline and each point during the follow-up period of the study. CFS was graded in each of the five corneal zones using the NEI grid (superior, nasal, central, inferior, temporal) in which a score of 0–3 (0 = normal and 3 = severe) was assigned to each of the five corneal regions in each eye. The maximum possible total score was 15 points per eye, with a total score of 0-3 classified as normal, 4-7 showing mild, superficial stippling, 8-11 having moderate, punctate staining, and a score of 12-15 as severe, with an epithelial defect. CFS responders were defined as those who had a 2-point total improvement from baseline to 6 months post-procedure. Extreme responders were classified as those that had an improvement in their category, i.e. an eye going from severe to mild or moderate to normal or any other combination that denotes improvement.
The Meibomian Gland Score (MGS) was used to measure meibomian gland function. A score was assigned after applying standardized pressure to an area of the lid including 5 glands. Scores ranged from 0 - 45 per eye and were assessed via an additive scale of 15 measurements per eye. The scale was anchored by 0 (no secretion) and 3 (clear liquid). We applied standard pressure using the Meibomian Gland Applicator (Johnson and Johnson) to the lower eyelids on both sides and documented secretion levels across the lids.
2.3.2. Secondary Endpoints
Participants were asked to assess their improvement after 1, 3, and 6 months of treatment, anchored by 0 (no difference) and 4 (significantly marked improvement). Participants were asked to assess their satisfaction after 1, 3, and 6 months of treatment, anchored by -2 (very dissatisfied) and 2 (very satisfied).
Statistical analyses were performed on all data using a one-sample t-test and repeated-subjects ANOVA, depending on the number of observations. A p-value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient Population
In all, 47 patients were recruited and completed treatment with the Forma-I handpiece, but not all subjects had complete data on all observations. When an observation was missing, the entire patient's data for that specific outcome was not included in the analysis. The study was performed at three separate institutions by three independent surgeons (Department of Plastic Surgery, Lenox Hill Hospital, New York, New York, USA, Department of Oculoplastic Surgery, Austin Face and Body, Austin, Texas, USA, and Excellent Vision Dry Eye and Rejuvenation Center in Portsmouth, NH, USA). These sites participated in the research, with measurements taken at baseline, 1-month, 3-months, and 6-months follow-up visits. The average patient age was 47.79 years, with the minimum age being 23 and the maximum age being 75 years. Of the 43 patients with gender data, 74% were female and 26% were male. Table 1 provides a summary of all primary endpoint measurements.
3.2. Standard Patient Evaluation of Eye Dryness (SPEED) Questionnaire
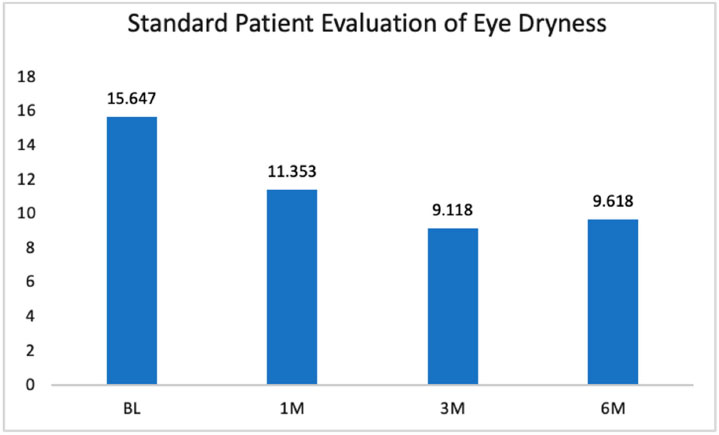
Thirty-two patients had complete baseline, 1-month, 3-month, and 6-month follow-up SPEED data. SPEED was assessed via a scale anchored by 0-4 (mild symptoms) and 8+ (severe symptoms). The within-subjects ANOVA was statistically significant, F(3, 99) = 23.42, p<0.001. The relationship was linear and consulting the pairwise comparisons demonstrated where those differences between periods occurred. 1-month, 3-month, and 6-month follow-up values were statistically lower than baseline values (Fig. 2). The 1-month values were also statistically higher than the 3-month values. In other words, SPEED improved from baseline to 1 month, and from 1 month to 3 months, before leveling off and maintaining that improvement through the 6-month follow-up period.
SPEED responders were those who had a 4 point improvement from baseline to the 6-month. Of the 32 patients with the 6-month data, 56% were responders. Additionally, extreme responders were also identified. This group of patients had severe dry eye symptoms at baseline and mild dry eye symptoms at 6 months. Only a few patients met this threshold (N = 2, or 6%).
| Item |
BL Mean |
1M Mean |
3M Mean |
6M Mean |
Improvement Stabilizes (based on p-value<0.05) |
|---|---|---|---|---|---|
| SPEED | 15.65 | 11.35 | 9.12 | 9.62 | Improves through 3M |
| OSDI | 34.50 | 25.15 | 21.88 | 23.59 | Improves through 1M |
| CFS Right | 2.74 | 0.38 | 0.34 | 0.17 | Improves through 1M |
| CFS Left | 3.27 | 0.41 | 0.53 | 0.09 | Improves through 1M |
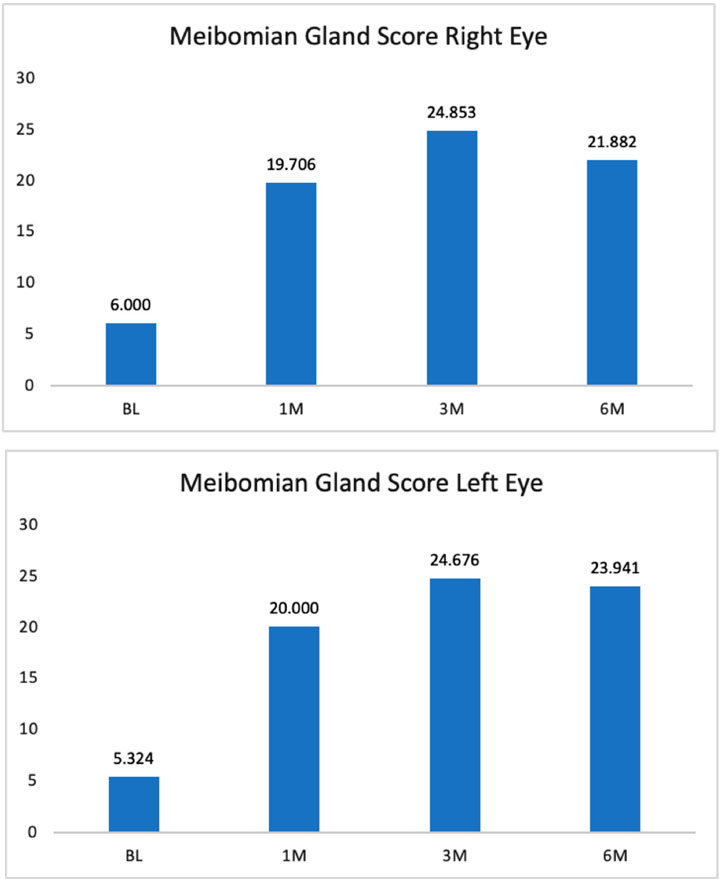
| MGS Right | 6.00 | 19.71 | 24.85 | 21.88 | Improves through 3M |
| MGS Left | 5.32 | 20.00 | 24.68 | 23.94 | Improves through 3M |
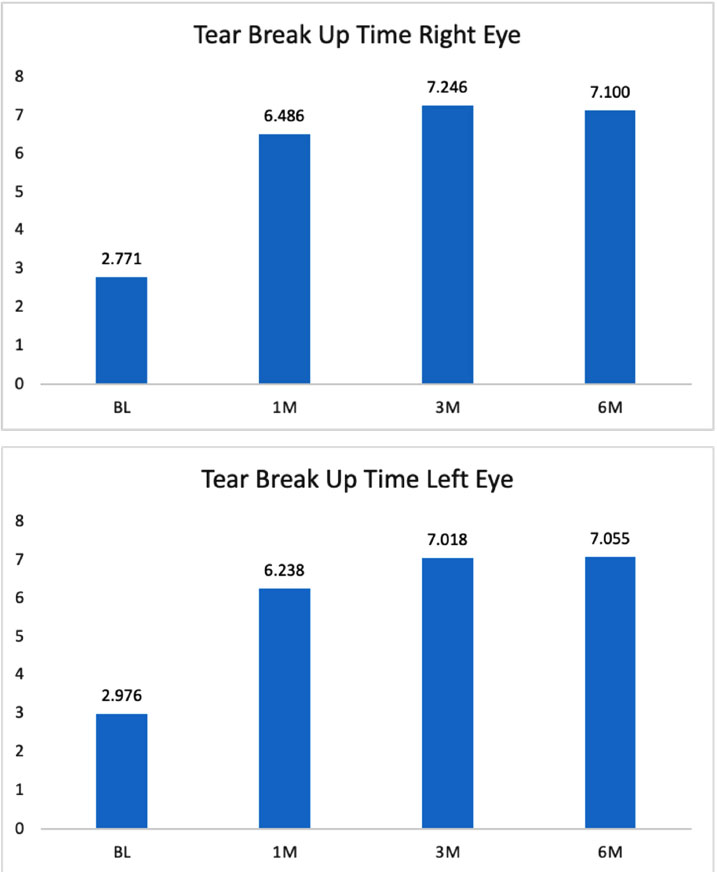
| TBUT Right | 2.77 | 6.49 | 7.25 | 7.10 | Improves through 1M |
| TBUT Left | 2.98 | 6.24 | 7.02 | 7.06 | Improves through 1M |
3.3. Ocular Surface Disease Index (OSDI)
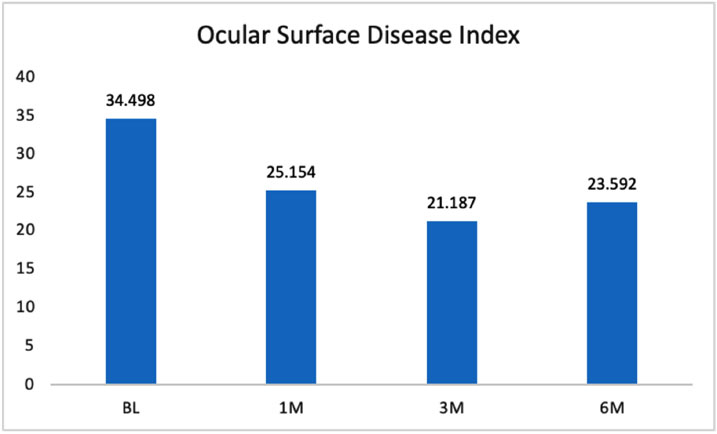
In this category, 35 patients had complete baseline, 1-month, 3-month, and 6-month follow-up data. ODSI was assessed via a scale anchored by 0-12 (normal) and 33+ (severe disease). The within-subjects ANOVA was statistically significant, F(3, 99) = 7.84, p = 0.01. The relationship was linear, and consulting the pairwise comparisons demonstrated where those differences among periods occurred. Follow-up values of 1-month, 3-month, and 6-month were statistically lower than baseline values; all follow-up visit results were statistically flat to one another. In other words, ODSI improved from baseline to 1 month, and this improvement was maintained through the 6-month follow-up period (Fig. 3).
ODSI responders were defined as those who had a 10-point improvement from baseline to 6 months. Of the 35 patients with 6-month follow-up data, 51% were responders. Additionally, extreme responders were also identified. This group of patients had severe dry eye symptoms at baseline and mild or normal dry eye symptoms at 6 months. Seventeen patients had severe symptoms at baseline, and 29% of those patients improved to mild or normal symptoms at 6 months.
3.4. Corneal Fluorescein Staining (CFS)
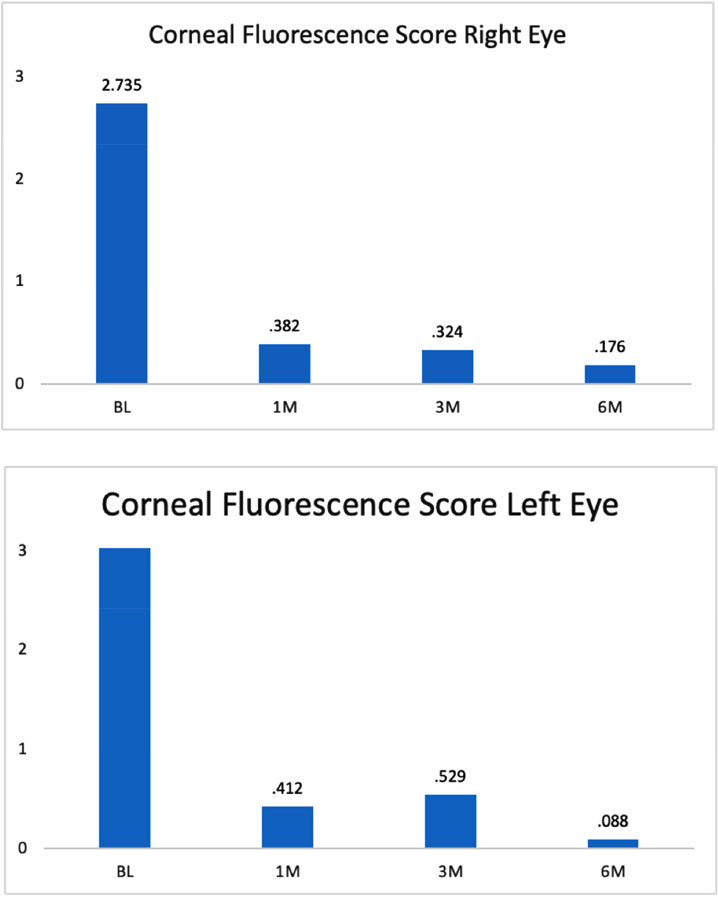
Thirty-five patients had complete baseline, 1-month, 3-month, and 6-month follow-up data. CFS was assessed via a scale anchored by 0-3 (normal) and 12-15 (epithelial defect or large coalescent erosion). The within-subjects ANOVA was statistically significant, F(3, 99) = 65.01, p<0.001. The relationship was linear, and consulting the pairwise comparisons demonstrated where those differences between periods occurred. Follow-up values of 1-month, 3-month, and 6-month were statistically lower than baseline values. Thus, the data showed that CFS right eye improved from baseline to 1-month post procedure this improvement was maintained at each follow-up visit (Fig. 4a).
CFS right eye responders were defined as those who had a 2-point improvement from baseline to 6 months. Of the thirty-five patients with 6-month data, 80% were responders. A subset of these patients was classified as extreme responders or those that had an improvement in at least one category from baseline to 6 months. Of the applicable 35 patients, 23% were extreme responders.
CFS metrics were similarly assessed for the left eye. Again, the within-subjects ANOVA was statistically significant, F(3, 99) = 36.26, p<0.001. The relationship was linear, and consulting the pairwise comparisons demonstrated where those differences between periods occurred. As seen with the right eye, for the left eye, 1-month, 3-month, and 6-month follow-up values were statistically lower than baseline values (Fig. 4b). Again, the data showed that CFS left eye improved from baseline to 1 month, and was maintained through 6 months of follow-up.
Of the thirty-one patients with CFS left eye 6-month data, 84% were considered responders. Additionally, extreme responders were also identified. An extreme responder is a patient that has an improvement of at least one category from baseline to 6 months. Of the applicable thirty-one patients, a total of 29% were extreme responders.
3.5. Meibomian Gland Score (MGS)
The MGS for the right eye was assessed via an additive scale of 15 measurements per eye; the scale was anchored by 0 (no secretion) and 3 (clear liquid). Thirty-five patients had complete baseline, 1-month, 3-month, and 6-month follow-up data. The within-subjects ANOVA was statistically significant, F(3, 99) = 53.59, p<0.001. The relationship was linear, and consulting the pairwise comparisons demonstrated where those differences between periods occurred. Follow-up values of 1- month, 3-month, and 6-month were statistically higher than baseline values (Fig. 5a). The 3-month values were also statistically higher than baseline and 1-month values, indicating that the MGS for the right eye improved from baseline to 1 month, and from 1 month to 3 months before stabilizing.
MGS responders were defined as those who had a 5-point improvement from baseline to 6 months post-procedure for the right eye. Of the thirty-five patients with 6-month data, 94% were considered responders.
Similar findings were observed for MGS in the left eye (Fig. 5b). Again, thirty-five patients had complete data at baseline and all follow-up visits. The within-subjects ANOVA was statistically significant, F(3, 99) = 45.21, p<0.001. The relationship was linear, and consulting the pairwise comparisons demonstrated where those differences between periods occurred. The MGS values were statistically higher at the 1, 3, and 6-month follow-up visits when compared to baseline. The 3 and 6-month values were also statistically higher than baseline and 1-month values, thus indicating that the MGS improved from baseline to 1 month and then from 1 month to 3 months. It was then stable from 3 months to 6 months after the procedure.
Responders for the left eye were again defined as those who had a 5-point improvement from baseline to 6 months. Of the thirty-five patients with 6-month data, 91% were responders.
3.6. Tear Breakup Time (TBUT)
TBUT measurements were assessed independently in the right (Fig. 6a) and left (Fig. 6b) eyes. For each eye, thirty-five patients had complete baseline, 1-month, 3-month, and 6-month follow-up data. TBUT was assessed via a scale anchored by less than 5s (dry eye) and greater than or equal to 10s (normal). The within-subjects ANOVA was statistically significant for the right eye, F(3, 99) = 20.63, p<0.001, and for the left eye F(3, 99) = 21.54, p<0.001. The relationship was linear in each and consulting the pairwise comparisons demonstrated where those differences between periods occurred. The data showed a statistically significant increase in TBUT at 1, 3, and 6 months after the procedure for both eyes. The TBUT improved from baseline to the 1-month visit and was still stable at the 3 and 6-month visits for both eyes.
TBUT responders were those who had at least a 3-second improvement from baseline to 6 months. Of the thirty-five patients with 6-month data, 60% were responders in the right eye and 69% were considered responders in the left eye. Extreme responders were identified as patients that had a TBUT of 5 seconds or less at baseline and a TBUT of 10 seconds or more at 6 months. Of the applicable 35 patients, 20% were extreme responders in the right eye and 17% in the left eye.
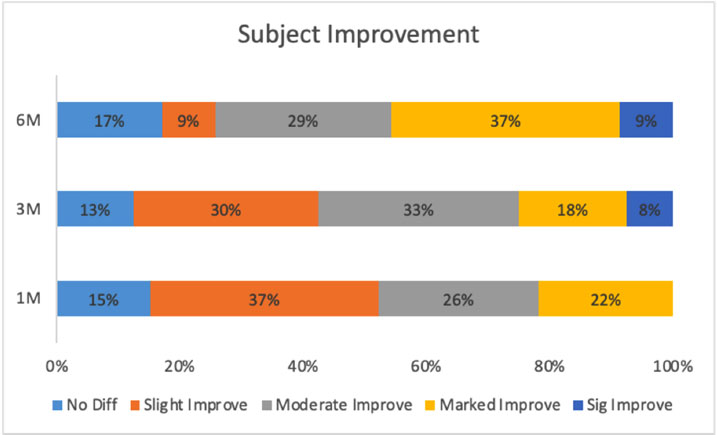
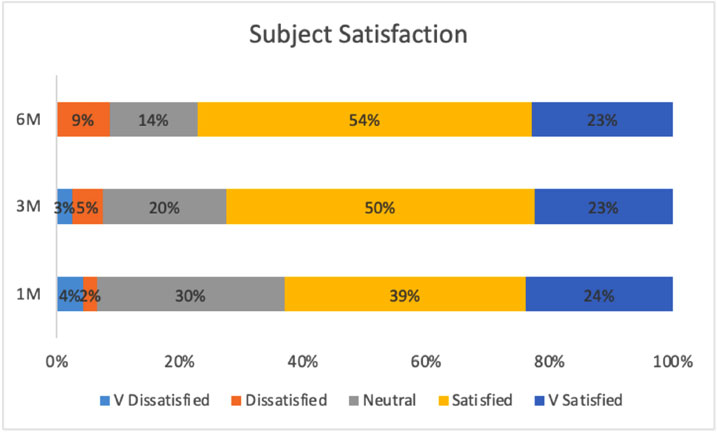
3.7. Subjective Improvement in Symptoms and Patient Satisfaction
Participants were asked to assess their improvement after 1, 3, and 6 months of treatment, anchored by 0 (no difference) and 4 (significantly marked improvement). The within-subjects ANOVA was statistically significant, F(2, 66) = 6.18, p = 0.01. Consulting the pairwise comparisons demonstrated that at 6 months, patients were more likely to believe they had improved as compared to 1 month and 3 months (Fig. 7). Additionally, all three improvement measurements were compared to the bottom value of the scale, 0, which indicates no improvement. All three median values were statistically higher than 0, which indicated that at each period, participants believed they saw some level of improvement. Taken together, participants were sufficiently satisfied with their improvement at 1-month, 3 months, and 6 months compared to believing there was no difference.
Participants were asked to assess their satisfaction after 1, 3, and 6 months of treatment, anchored by -2 (very dissatisfied) and 2 (very satisfied). The within-subjects ANOVA was not statistically significant, F(2, 66) = 0.85, p = 0.43. Additionally, all three improvement measurements were compared to the middle value of the scale, 0, which indicates neutral satisfaction. All three mean values were statistically higher than 0, which indicated that at each period, participants believed they were satisfied beyond a neutral level (Fig. 8). Taken together, participants were sufficiently satisfied with their improvement at 1 month and maintained that perception of improvement at 3, and 6 months.
3.8. Complications
For a follow-up period of 6 months, there were no skin burns or corneal trauma. Patients noted mild erythema of the periorbital skin that resolved in 24-48 hours. There were no complaints of a burning sensation during the procedure. No treatment was abandoned as no intolerance or excessive thermal sensation was reported.
4. DISCUSSION
The purpose of this study is to evaluate the safety and efficacy of a novel, in-office treatment protocol for meibomian gland dysfunction (MGD). MGD is a major burden on the healthcare system, with up to 78% of patients who consult an eye care provider suffering from symptoms of this disease [4, 5]. Current remedies include at-home warm compresses, lid massage, oral tetracyclines, and several in-office procedures. The at-home remedies suffer from a lack of patient compliance as they are rather burdensome, requiring twice or more daily applications of warm compresses, somewhat painful lid massage, or antibiotics with several side effects [6]. Over the past decade, several in-office procedures have been introduced to try to reduce patient suffering by mitigating these compliance issues. Most of these treatments involve the application of heat to the eyelid to melt the thickened meibomian gland secretions, followed by manual or machine-aided gland expression. Several studies have been done to evaluate the efficacy of these treatment modalities with varied results.
The thermal pulsation system Lipiflow, which is a device with a single-use applicator for each eyelid, has been used as a “hands-free” device that is enabled to treat meibomian gland disease [7]. The device treats the eyelids with heat and pressure that is regulated by sensors that increase gland secretion, is being utilized in both ophthalmology and optometry clinics, and uses a proprietary technology called Vectored Thermal Pulse. Along with force equalization, the eye is protected from heat and pressure while a nominal therapeutic temperature of 42.5 degrees celsius is applied directly to the inner eyelid where the glands are located while protecting the eyelid or delicate structures of the globe. Although many patients will see a change in symptoms right away, results generally optimize over six to eight weeks. The company states that results can last up to two years and they have recently re-engineered the device intending to enable more efficient and accurate positioning in the eyes, allowing for a more even application of heat and pressure across the entire eyelid [8, 9]. The shortcoming of this device is that it is still dependent on patient positioning and does not allow a customized treatment plan.
Intense Pulsed Light (IPL) has emerged as a potential treatment for dry eye over the past several years. Initial anecdotal evidence from cosmetic clinics, where patients were having IPL performed for rosacea, showed that symptoms of dry eye could be reduced by the treatment [10]. Building upon this, controlled trials were undertaken to assess the validity of this treatment for dry eye disease. In a recent, multicenter randomized controlled trial, IPL with physical gland expression was compared to sham IPL with gland expression [11]. The authors found a significant improvement in TBUT and MGS but did not see a statistically significant difference in OSDI. Another study of 87 patients compared IPL with placebo across several parameters [12]. Significant improvements in both OSDI and SPEED were noted out to the study endpoint of 75 days. A meta-analysis of nine studies involving IPL was performed and published in 2021. The authors included 539 patients in the analysis from the nine studies and examined outcomes such as SPEED, OSDI, and TBUT. The results showed that IPL with meibomian gland expression resulted in improvements in TBUT and OSDI, but not SPEED [13]. They concluded that IPL alone is not superior to meibomian gland expression, but the combination of the two can reduce symptoms. They noted that three or four sessions are needed to show efficacy lasting up to 6 months.
In our study, we found that a single RF treatment using the Forma-I handpiece combined with meibomian gland expression resulted in significant improvement across all endpoints including, SPEED, OSDI, TBUT, CFS, and MGS. No additional treatments were performed during the follow-up period. Additionally, patients felt satisfied with the treatment and thought that it resulted in improvement. The treatment itself could be delegated to a mid-level provider after a training session and observed by the physician to verify the technique. It is performed comfortably without the need for topical anesthetic or a delay in treatment time. The treating physician did not have to place corneal shields on the eyes as might be needed for IPL treatments. Additionally, there is no consumable cost such as with Lipiflow, where a costly, disposable activator must be used for each treatment session. This helps to reduce the cost of the procedure for the patient and the treating physician.
We also observed the potential for combination treatment with IPL, which treats at different cellular levels for dry eye and meibomian gland disease. Interestingly, not only is there potential for medical improvement but there is also an improvement in skin quality, texture, and tone. Finally, a key component of treatment was the expression of the glands. These clinical observations are promising from this pilot study that there is a reliable alternative treatment available for chronic dry eye patients.
CONCLUSION
This pilot study examined the safety and efficacy of RF-mediated meibomian gland expression for the treatment of dry eye symptoms. The data supports the conclusion that the Forma-I treatment was efficacious in treating dry eye symptoms. Patients believed the treatment significantly improved from their baseline and reported high satisfaction. Also, no complications were reported within the follow-up period. Across all endpoint metrics, the treatment was considered a success in treating dry eye symptoms.
LIST OF ABBREVIATIONS
| IPL | = Intense Pulsed Light |
| MGD | = Meibomian Gland Dysfunction |
| TBUT | = Tear Breakup Time |
| ANOVA | = Analysis of Variance |
| RF | = Radiofrequency |
| SPEED | = Standard Patient Evaluation of Eye Dryness |
| OSDI | = Ocular Surface Disease Index |
| TBUT | = Tear Breakup Time |
| CFS | = Corneal Fluorescence Score |
| MGS | = Meibomian Gland Score |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by Sterling Institutional Review Board) (REF#7546-D0609175A).
HUMAN AND ANIMAL RIGHTS
No animals were used for the study.All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all the patients.
AVAILABILITY OF DATA AND MATERIAL
All the data and supportive information is provided within the article.
STANDARDS OF REPORTING
STROBE guidelines were followed.
FUNDING
This study was funded by InMode.
CONFLICT OF INTEREST
The authors declare no conflicts of interest financial or otherwise.
ACKNOWLEDEGEMENTS
Declared none.


