RESEARCH ARTICLE
Performance of a Silicone Hydrogel Daily Disposable Contact Lens among Wearers with Lens-related Dryness
William Reindel1, Robert Steffen1, Gary Mosehauer1, Jeffery Schafer1, Marjorie Rah1, *, Ayda Shahidi1, Howard Proskin2
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187436412303021
Publisher ID: e187436412303021
DOI: 10.2174/18743641-v17-230316-2022-57
Article History:
Received Date: 07/10/2022Revision Received Date: 07/02/2023
Acceptance Date: 16/02/2023
Electronic publication date: 31/03/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Addressing contact lens dryness continues to be a development goal of contact lens (CL) manufacturers.
Objective:
The objective of this study is to evaluate the clinical performance of kalifilcon A, a daily disposable silicone hydrogel (SiHy) CL, in subjects that experience dryness with their habitual planned-replacement SiHy CLs relative to a non-dry subgroup.
Methods:
A cohort of adapted planned-replacement SiHy CL wearers wore kalifilcon A lenses for at least 8 hours daily over two weeks. After one week of lens wear, subjects completed a survey regarding their lens wearing experience with respect to comfort and vision. Subsequently, subjects visited the clinics for the 2-week visit, during which the investigators completed a slit lamp exam and questionnaire regarding lens performance.
Results:
The evaluation included 180 subjects experiencing CL dryness with their habitual SiHy lenses and 213 subjects that did not. Both subgroups largely agreed with all comfort and vision attribute statements, and the dryness subgroup expressed higher levels of agreement with most comfort-related statements. Among habitual rewetting drop users, 73.9% in the dryness subgroup and 73.1% in the non-dry subgroup used drops less frequently while wearing kalifilcon A lenses. Investigators found no > Grade 2 slit-lamp findings, nor differences between subgroups. Neither subgroup showed a change in ratings between visits, except for a significantly higher proportion of improvers in the non-dry subgroup for upper lid tarsal conjunctival abnormalities.
Conclusion:
The kalifilcon A lens performed well among habitual planned-replacement SiHy CLs wearers. Its unique chemistry can provide a more satisfying wear experience for SiHy lens wearers experiencing CL dryness.
1. INTRODUCTION
Silicone hydrogel (SiHy) contact lenses (CLs) account for more lens fits than all other lens material types combined [1]. Because CL dryness is one of the primary factors which leads to decreased lens wear time [2] or discontinuation of lens wear altogether [2-7], reducing dryness symptoms is an important goal for CL product development. There are many factors that can contribute to the dry eye condition, described as a loss of homeostasis at the ocular surface. Instability of the tear film and hyperosmolarity, inflammation, and neurosensory anomalies all play roles. For CL wearers who experience dry eye symptoms, biological changes include poor wettability due to a thinner, patchy lipid layer, instability of the tear film, increased tear evaporation rate, reduced turnover rate of basal tears, reduced tear meniscus volume, and increased tear osmolarity [8].
To mitigate the symptoms of CL-related dryness, numerous strategies have been suggested; these include fitting with daily disposable (DD) lenses, fitting of lenses fabricated with internal wetting agents, topical application of lubricating wetting agents, nutritional supplementation of omega-3 and omega-6 fatty acids, antibiotic therapy, and reducing wearing time or ceasing lens wear altogether [8]. As CL-related dryness continues to be reported by wearers of all types of CL [9], additional strategies beyond lens material and design are necessary to offer wearers a more satisfying lens wear experience.
Inspired by the Tear Film & Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II Management and Therapy Report [10], the kalifilcon A silicone hydrogel CL (Bausch & Lomb Incorporated, Rochester NY) [11] is infused with a combination of ingredients during the manufacturing process [12]. Because the kalifilcon A DD silicone hydrogel lens material chemistry, in combination with its infused solution components, represents an advancement in CL technology, it is important to evaluate the clinical performance and patient experience among a diverse group of CL wearers. This study represents the first real-world large-scale evaluation of the kalifilcon A DD silicone hydrogel lens. The purpose of this evaluation was to assess the clinical performance of the kalifilcon A CL in order to compare the comfort and vision experiences of subjects with and without CL-related dryness with their habitual SiHy CLs.
2. MATERIALS AND METHODS
A total of 36 investigative sites in the United States participated in this two-week study from July 31 - October 9, 2019. All sites obtained Institutional Review Board (IRB) approval from Sterling Institutional Review Board (Atlanta, GA), and potential participants provided written informed consent prior to the determination of eligibility to participate in the study. Subject recruitment was open to subjects who met all of the inclusion criteria and none of the exclusion criteria.
An analysis of data from a cohort of subjects between the ages of 18-40 years and who wore kalifilcon A CLs was conducted. To participate in the study, these subjects were required to have had their eyes examined by an eye care practitioner within the two years preceding the study and habitually wore planned replacement SiHy, single-vision CLs, including: lotrafilcon B (Air Optix Aqua or Air Optix with HydraGlyde, Alcon, Fort Worth, TX), lotrafilcon A (Air Optix Night & Day, Alcon), balafilcon A (PureVision2, Bausch & Lomb Incorporated), samfilcon A (Bausch + Lomb ULTRA, Bausch & Lomb Incorporated), comfilcon A (Biofinity, CooperVision, Pleasanton, CA), senofilcon A (Acuvue Oasys with HydraClear, Johnson & Johnson Vision, Jacksonville, FL), and senofilcon C (Acuvue Vita, Johnson & Johnson Vision). The study population was selected such that approximately equal fractions (one-quarter) of subjects habitually wore lenses from each of the four manufacturers. Subjects were excluded if they had a history of corneal or refractive surgery or were using ocular medication or any systemic medication that would affect ocular physiology. Subjects that habitually wore DD, monovision, multifocal, or toric CLs, as well as those with ≥ Grade 2 findings during the eligibility slit lamp examination also were excluded from the study.
All participants underwent a thorough slit lamp examination performed by the investigators at the screening/dispensing and follow-up visits. Slit lamp signs of epithelial edema, epithelial microcysts, limbal injection, bulbar injection, corneal infiltrates, corneal neovascularization, upper lid tarsal conjunctival abnormalities, and corneal staining were graded using an ordinal, text-based scale, from which numeric grades in integer steps were assigned, 0 (no finding), 1 (trace), 2 (mild), 3 (moderate) and 4 (severe). Fluorescein corneal staining grades were computed as the maximum grade taken within each of five different corneal locations (central, inferior, nasal, superior, and temporal), and the worst case was used as the single corneal staining grade. Only those subjects that presented with 0 (no finding) or 1 (trace finding) at the dispensing visit were enrolled in the study. All investigators and their site staff were trained to ensure consistent standard procedures amongst sites were used for grading the findings.
At the dispensing visit, subjects were fitted with kalifilcon A DD silicone hydrogel CLs bilaterally. Subjects were dispensed a 2-week supply of lenses and were instructed that other contact lenses (except the study lenses) were not allowed to be used during the study period. In addition, Bausch + Lomb Sensitive Eyes Drops were provided for use as needed.
After a minimum of 7 days of lens wear (in all cases prior to the 2-week follow-up visit), subjects directly reported their prior wearing experience, time spent on various activities, and performance of the test lens by completing an online questionnaire. Subjects rated performance using a 6-point Likert scale, where scores were assigned to each subject’s responses: 6 = strongly agree; 5 = agree; 4 = slightly agree; 3 = slightly disagree; 2 = disagree; and 1 = strongly disagree. This survey is similar to other patient reported outcomes questionnaires available in the literature [13-15].
Following two weeks of lens wear, subjects returned to their respective dispensing clinics for a follow-up/exit examination. During this visit, the investigators completed a questionnaire regarding lens fit and their overall impression, using numerical scores of 5 = completely satisfied/excellent; 4 = very satisfied/very good; 3 = somewhat satisfied or good; 2 = not very satisfied or fair; and 1 = not at all satisfied or poor, as well as two lens performance attributes (lens delivers clear vision and lens helps maintain a smooth, wettable surface) using the 6-point Likert scale for agreement as described above.
As part of the survey, subjects provided ratings associated with their habitual SiHy lenses, including whether or not they experience CL dryness symptoms. Those who indicated that their eyes were very dry or somewhat dry during wear of their habitual lenses were placed in the dryness subgroup, while those who indicated that their eyes were not very dry or not at all dry were placed in the non-dry subgroup. The dryness designation did not imply a clinical dry eye diagnosis and was focused on subject perception of CL-related dryness.
Frequency of rewetting drop use with habitual lenses was recorded for each subject at screening, and the corresponding frequency of use with the kalifilcon A lens was recorded at the 2-week visit.
2.1. Statistical Methods
Subjects’ age, gender and CL wear durations (daily and weekly) were compared based on the two-sample t-test or chi-squared test where appropriate.
For each comfort and vision attribute included in the survey, each subject was categorized as either being in agreement (top 3 boxes) or in disagreement with the attribute. The proportion of subjects indicating agreement for the attribute was tabulated for the dryness and non-dry subgroups, and the ratio of these respective proportions (relative risk, RR) was calculated, along with an associated 95% confidence interval (for RR) using the Wald method. This approach was used to illustrate better the relative differences between the dryness and non-dry subgroups. An RR value of 1 indicates a comparable outcome of the positive characteristics associated with wearing kalifilcon A lenses; values > 1 are favorable to the dryness subgroup; and values < 1 are favorable to the non-dry subgroup. The agreement proportions were compared between the subgroups using the Wald test.
Investigator ratings for lens fit, performance, and satisfaction were similarly summarized and compared between the dryness and non-dry subgroups.
Analyses of the graded slit lamp parameters were based on subject-wise scores obtained for each examination by taking the highest of the scores evaluated on the subject’s right and left eyes (as eye-wise scores do not represent independent sampling units). The distributions of these subject-wise scores at both the dispensing and 2-week visits for each of the graded slit lamp parameters were compared between subgroups using the Mann-Whitney U test. For subjects exhibiting a change in score between the dispensing and 2-Week visits, an indicator variable for improvement (0 = score worsened, 1 = score improved) was created for each slit lamp parameter and compared between the subgroups using a Wilcoxon signed-rank test.
Analyses of the reported frequencies of rewetting drop usage were based on data from subjects that reported using rewetting drops while wearing habitual lenses. Each subject was categorized according to whether he or she used rewetting drops less frequently while wearing kalifilcon A lenses during the study than while wearing habitual lenses, or with the same frequency, or with greater frequency. The proportions of subjects that reported a reduction in the frequency of rewetting drop use during the study were compared between the dryness subgroup and the non-dry subgroup using a Fisher’s exact test.
A significance level of α = 0.05 was utilized for all statistical hypothesis tests.
3. RESULTS
3.1. Subject Demographics
Data from 393 subjects were analyzed. The subjects were classified into dryness and non-dry subgroups based on their responses to the patient questionnaire. Baseline demographics for the 393 subjects that completed the survey are presented in Table 1. A total of 46% of subjects reported experiencing CL dryness with their habitual SiHy lenses and were classified into the dryness subgroup; 53% did not and were thus classified into the non-dry subgroup. In both the dryness and non-dry subgroups, all but one subject completed the two-week study.
At screening, there was no difference between dryness and non-dry subgroups with respect to age, nor baseline-reported duration or frequency of CL wear, both in terms of hours per day and days per week that lenses were worn (all p ≥ 0.05). Females were more likely than males to experience dryness (p < 0.05), consistent with previous reports [16]. Those in the dryness subgroup were more likely than those in the non-dry subgroup to use rewetting drops habitually (p < 0.05).
| - | Population | - | ||
|---|---|---|---|---|
| Characteristic |
Dryness Subgroup (N=180) |
Non-dry Subgroup (N=213) | p-value#,§ | |
| Age | Years (Avg ± Sdev) | |||
| - | 30.6±5.63 | 30.4±5.72 | 0.73# | |
| Gender | % (n) | |||
| Female | 71.7 (129) | 60.6 (129) | 0.02§,* | |
| Male | 28.3 (51) | 39.4 (84) | ||
|
Habitual Rewetting Drop User |
% (n) | |||
| Yes | 38.3 (69) | 12.2 (26) | p<0.0001§,* | |
| No | 61.7 (111) | 87.8 (187) | ||
| Habitual Lens Wear | Duration (Hours or Days) | - | ||
| Daily (Hours) | 14.0±3.44 | 14.3±3.82 | 0.42# | |
| Weekly (Days) | 6.6±0.86 | 6.7±0.68 | 0.20# | |
# Between-group p-value comparing mean scores based upon two-sample t-test.
* Significant at p < 0.05.
| - | Population | - | |
|---|---|---|---|
| Device or Media |
Dryness Subgroup (N=180) |
Non-dry Subgroup (N=213) | p-value# |
| - | Duration (Hours) | - | |
| Office computer | 5.6±4.6 | 5.3±4.4 | 0.51 |
| Home computer | 2.0±3.0 | 1.8±2.2 | 0.45 |
| Television | 2.6±2.2 | 2.6±2.5 | >0.99 |
| Smartphone, tablet | 4.9±4.8 | 4.5±4.2 | 0.38 |
| Book, magazine, newspaper | 1.4±1.7 | 1.5±2.4 | 0.64 |
| Electronic game | 0.8±1.8 | 0.8±1.6 | >0.99 |
The average daily wear times of the kalifilcon A study lenses over the 2-week period did not differ between subgroups (p ≥ 0.05). Similarly, the average number of days per week that the study lenses were worn also did not differ between subgroups.
Average daily use of digital devices and reading materials is summarized in Table 2. The most common digital devices used were computers and smartphones or tablets. There was no difference between subgroups with respect to the duration of use of any of the queried devices or media (all p ≥ 0.05).
3.2. Patient Reported Outcomes for Comfort and Vision
The patient questionnaire assessed 12 attributes associated with comfort and 8 attributes associated with vision. For each of these attributes, favorable ratings (agreement with the attributes) were reported by the majority of subjects in both the dryness and non-dry subgroups.
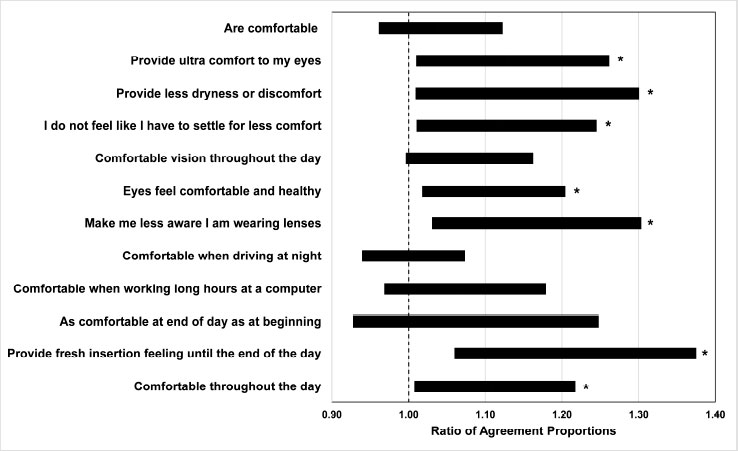
The 95% confidence intervals for the RRs comparing subgroups with respect to the proportions of subjects presenting positive comfort outcomes are illustrated in Fig. (1). For over half of the queried comfort attributes, a greater proportion of favorable findings was indicated in the dryness subgroup than in the non-dry subgroup, as evidenced by an RR significantly greater than 1 (all p < 0.05).
Corresponding results for the vision outcomes are presented in Fig. (2). Of the vision attributes, only “delivered exceptional clarity and comfort” differed between subgroups and favored the dryness subgroup (p < 0.05).
3.3. Slit Lamp Examination Results
Nearly all slit lamp examinations at both the dispensing and 2-week visits were graded as no finding (Grade 0) or trace (Grade 1) for both subgroups. Only a single subject in the dryness subgroup presented with a mild (Grade 2) upper lid tarsal conjunctival abnormalities finding, while the non-dry subgroup presented with mild (Grade 2) corneal infiltrates in one subject, corneal staining in two subjects, and upper lid tarsal conjunctival abnormalities in one subject. Neither subgroup presented with any finding greater than Grade 2 (moderate or severe finding). There was no difference between subgroups in the distribution of scores at either visit for any attribute (all p ≥ 0.05).
| Parameter | p-value# | Number of Subjects Whose Score Changed between Baseline and 2wk visits | ||
|---|---|---|---|---|
| Improved | Worsened | Total | ||
| Bulbar injection | 0.09 | 10 | 3 | 13 |
| Corneal infiltrates | >0.99 | 0 | 1 | 1 |
| Corneal neovascularization | >0.99 | 1 | 0 | 1 |
| Corneal staining | 0.83 | 10 | 11 | 21 |
| Epithelial edema | >0.99 | 1 | 0 | 1 |
| Epithelial microcysts | NA | 0 | 0 | 0 |
| Limbal injection | >0.99 | 6 | 5 | 11 |
| Upper lid tarsal conjunctival abnormalities | >0.99 | 6 | 6 | 12 |
# p-value from Wilcoxon Signed-Rank test comparing indicators for improvement versus worsening.
| Parameter | p-value# | Number of Subjects Whose Score Changed between Baseline and 2wk visits | ||
|---|---|---|---|---|
| Improved | Worsened | Total | ||
| Bulbar injection | 0.69 | 13 | 11 | 24 |
| Corneal infiltrates | >0.99 | 0 | 1 | 1 |
| Corneal neovascularization | >0.99 | 1 | 0 | 1 |
| Corneal staining | 0.88 | 22 | 21 | 43 |
| Epithelial edema | NA | 0 | 0 | 0 |
| Epithelial microcysts | >0.99 | 2 | 1 | 3 |
| Limbal injection | 0.63 | 7 | 10 | 17 |
| Upper lid tarsal conjunctival abnormalities | 0.04* | 14 | 3 | 17 |
# p-value from Wilcoxon Signed-Rank test comparing indicators for improvement versus worsening.
* Significant at p < 0.05.
Summaries of the changes in graded slit lamp findings between dispensing and 2-week visits in the dryness and non-dry subgroups are presented in Tables 3 and 4, respectively. For each parameter, the number of subjects that presented score improvements and score worsenings (as well as the sum of these) are reported. The remainder of the subjects in the summarized subgroup presented the same score at both visits. For the dryness subgroup, among subjects exhibiting different scores between visits, no significant difference was observed between the proportion of subjects who exhibited improvements and the proportion who exhibited worsenings for any graded slit lamp parameter (all p ≥ 0.05). For the non-dry subgroup, the corresponding analyses presented the same findings except for upper lid tarsal conjunctival abnormalities, for which a significantly higher proportion of improvers was indicated (p < 0.05).
3.4. Self-reported Use of Rewetting Drops
At screening, a greater percentage of subjects in the dryness subgroup compared with the non-dry subgroup reported the use of rewetting drops while wearing their habitual lenses (p<0.05). Among these, 38 (55.1%) in the dryness subgroup and 16 (61.5%) in the non-dry subgroup reported that they did not use the rewetting drops while wearing the kalifilcon A lenses during the study. Among the habitual rewetting drop users in the dryness subgroup, 51 subjects (73.9%) reported a reduction in the frequency of rewetting drop use while wearing kalifilcon A lenses during the study; 7 (10.1%) reported the same frequency of use; and 11 (15.9%) reported an increase in the frequency of use. The corresponding changes in usage frequencies among habitual rewetting drop users in the non-dry subgroup were 19 (73.1%), 6 (23.1%), and 1 (3.8%), respectively. The two subgroups did not differ with respect to the proportions of subjects who reported a reduction in the frequency of rewetting drop use (p>0.05).
3.5. Investigator Questionnaire Results
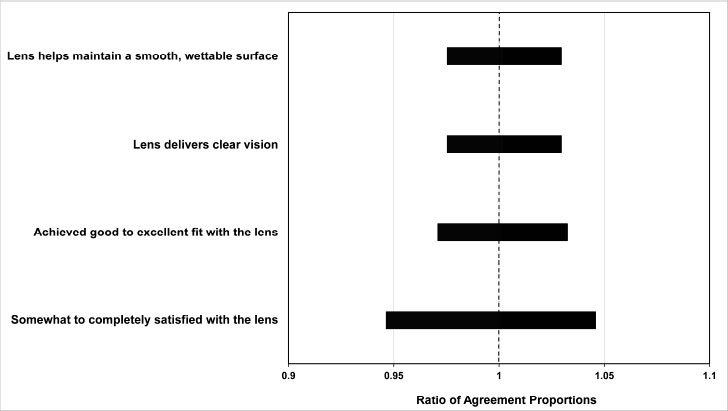
Investigator questionnaire outcomes indicated a high level of overall satisfaction with the kalifilcon A lens. The relative comparison of investigator agreement for each lens attribute statement is shown in Fig. (3). There was no difference between dryness and non-dry subgroups with respect to investigator agreement for maintaining a smooth wettable surface, clear vision, fit and satisfaction.
4. DISCUSSION
CL-related dryness continues to be reported as a common symptom associated with CL wear [9]. A 2019 survey of DD silicone hydrogel lens wearers found that 53% reported CL dryness [17]. New strategies for addressing CL-related dryness can be derived from the evaluation of the complex dry eye condition. TFOS DEWS II described dry eye as a multifactorial disease of the tear film and ocular surface, with loss of tear film homeostasis as a common cause [18]. It is recognized that CL wear can contribute to these signs and symptoms by challenging tear film stability [19], increasing tear osmolarity [10, 20], and mediating ocular inflammation [19, 21-23]. These phenomena can be related, as tear evaporation causes both tear instability and hyperosmolarity, which stresses the corneal epithelium [24] and can lead to CL-induced dry eye. While studies report that osmolarity is elevated to a greater degree in those experiencing dry eye [25, 26], measurements using tear fluid sampled from the meniscus can underestimate the true osmolarity of the pre-lens tear film [27] and miss specific areas of extreme hyperosmolarity due to localized tear breakup [28, 29].
Sustaining compatibility of the CL with the ocular surface remains a fundamental goal in lens development to help reduce symptoms of dryness and discomfort. However, as lens materials and designs have advanced, symptoms of CL dryness have persisted [17], and alternate strategies for maintaining ocular surface homeostasis are needed. Limited attention has been given to the integration of solution components into the lens during the manufacturing process.
Over the years, the inclusion of various ingredients in artificial tears, such as viscosity enhancers, humectants, lubricants/moisturizers, and combinations thereof [30], has been shown to alleviate dry eye symptoms. Similar approaches have been applied for integrating ingredients into CL care solutions, with the intention to lubricate and moisturize CLs. Pre-treatment of lenses with poloxamine 1107 polymeric surfactant was demonstrated to increase wetting of the CLs due to surfactant adsorption that persists over at least eight hours of wear, resulting in more subjective comfort [31]. In addition to ocular lubricants, other ingredients, including osmoprotectants such as l-carnitine [32-37], erythritol [32-39], betaine [32, 36, 37], and glycerin [33-35, 37-40] that help preserve normal tear tonicity [41], as well as non-sodium electrolytes such as potassium [42-44], have been implicated as contributors to maintaining ocular surface homeostasis and potentially alleviating CL-related dryness.
The kalifilcon A SiHy material was developed with lens design properties intended to help maintain ocular surface homeostasis. Kalifilcon A polymerizes in two time resolved phases, with methacrylate monomers polymerizing first to form the silicone backbone and n-vinyl pyrrolidone (NVP) polymerizing to form high molecular weight polyvinylpyrrolidone (PVP) surrounding the silicone [45]. The material characteristics offer high oxygen transmissibility with a low modulus (0.50 MPa) and high nominal water content (55%) [12]. The lens was designed with sufficient ionic permeability to allow for passive ion diffusion, as well as proper hydrophilic character to retain moisture.
Concentration of tear film components, such as sodium ions leading to hyperosmolarity can disturb ocular surface homeostasis [46, 47]. Diffusion of beneficial tear components such as essential ions, antioxidants, and osmoprotectants can contribute to the maintenance of ocular surface homeostasis. As such, the kalifilcon A material is infused during the manufacturing process with a solution that includes dual lubricating/moisturizing polymeric surface active agents (surfactants, poloxamine 1107 and poloxamer 181), as well as an additional moisturizer (glycerin, a synthetic polyol demulcent used in numerous ophthalmic preparations that also acts as an osmoprotectant), additional osmoprotectant (erythritol, a natural polyol) [48], and the electrolyte potassium in a phosphate-based buffering system [12]. The combination of lens material with infused ingredients is designed to help maintain ocular surface homeostasis and provide a positive wearing experience.
This investigation provides the first large-scale, real-world evaluation of the clinical performance of the kalifilcon A DD silicone hydrogel CL, which is infused with a combination of ingredients inspired by the DEWS II Management and Therapy Report [10] among a group of habitual planned-replacement SiHy CL wearers that reported experiencing CL-related dryness and a group that did not report dryness associated with wear of their habitual lens. Within the study population, 46% of subjects reported CL-related dryness with their habitual SiHy lens, which is consistent with previous reports in the literature [9, 49]. Overall, the kalifilcon A lens performed well, as indicated by both dryness and non-dry subgroup survey results and investigator survey results. Subjects placed in the dryness subgroup found the lens to perform especially well, as they reported higher comfort-related quantitative agreement ratings than did the non-dry subgroup.
Slit lamp findings at the 2-week visit were unremarkable. The dearth of Grade 2 or greater findings and relative lack of changes in scores for all measured slit lamp parameters suggests that the results are in concordance with the kalifilcon A lens maintaining ocular surface homeostasis over the wearing period. Maintenance of homeostasis during kalifilcon A lens wear was also supported by reduced usage of rewetting drops by participants that applied these habitually. Instructed, “You may use these drops as needed throughout the study,” 73.9% of habitual rewetting drop users in the dryness subgroup reported a reduction in the frequency of rewetting drop use while wearing kalifilcon A lenses during the study; there was a similar reduction among habitual rewetting drop users in the non-dry subgroup (73.1%). Most participants chose not to use supplemental rewetting drops at all while wearing the kalifilcon A lens, regardless of dry eye status.
The study investigators also indicated that the lens performed well. They were satisfied overall with the lens, found the lens fit to be good, and agreed that the lens delivers clear vision and helps maintain a smooth, wettable surface (Fig. 3). The investigator survey results indicate no difference between subgroups with respect to these lens performance attributes.
Wearer and investigator satisfaction with kalifilcon A DD silicone hydrogel CLs might be attributed to ingredients infused into the lens; however, the material characteristics of the lens itself, including oxygen transmissibility, wettability, and relatively low modulus also contribute to the lens performance. Collectively, the study findings support the science behind infusion of beneficial ingredients into a DD silicone hydrogel CL. The specific lens properties and infused solution components that most contribute to generally higher comfort scores in the dryness subgroup are not entirely known and remain to be explored in future studies. In addition to the study-specific questionnaires used in this study, other standard questionnaires reported in the literature may be valuable for use in subsequent evaluations.
CONCLUSION
The kalifilcon A CL offers wearers and eye care practitioners an innovative DD silicone hydrogel lens. Its unique chemistry and infused ingredients, based upon insights from the TFOS DEWS II Management and Therapy Report [10], provided current SiHy CL wearers that reported dryness with their habitual lenses a more satisfying wear experience than those who did not report dryness with their habitual lenses as shown in this study.
LIST OF ABBREVIATIONS
| SiHy | = Silicone Hydrogel |
| CLs | = Contact Lenses |
| DD | = Daily Disposable |
| TFOS | = Tear Film & Ocular Surface Society |
| DEWS | = Dry Eye Workshop |
| IRB | = Institutional Review Board |
| RR | = Relative Risk |
| NVP | = N-Vinyl Pyrrolidone |
| PVP | = Polyvinylpyrrolidone |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the respective study site IRBs and the Sterling Institutional Review Board (Atlanta, GA).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
All subjects enrolled in the study participated voluntarily after reading and signing a previously written informed consent.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The datasets reported in and analyzed during the current study are not publicly available due to their proprietary nature. No data beyond those disclosed herein will be shared.
FUNDING
This work was funded by Bausch & Lomb Incorporated.
CONFLICT OF INTEREST
Howard Proskin is a consultant to Bausch & Lomb Incorporated.
All other authors are employed by Bausch & Lomb Incorporated.
ACKNOWLEDGEMENTS
The abstract of this paper was presented at the American Academy of Optometry meeting, October 7-22, 2020, as a poster presentation (Board 285 205283). The poster’s abstract was published online and is available for download from: https://aaopt.org/past-meeting-abstract-archives/?SortBy=&ArticleYear=2020&ArticleAuthor=REINDEL. [cited: 10th Sep 2022]
REFERENCES
| [1] | Morgan PB, Woods CA, Tranoudis IG, et al. International contact lens prescribing in 2022. Contact Lens Spectr 2023; 38(1): 28-35. |
| [2] | Begley CG, Caffery B, Nichols KK, Chalmers R. Responses of contact lens wearers to a dry eye survey. Optom Vis Sci 2000; 77(1): 40-6. |
| [3] | Sindt CW, Longmuir RA. Contact lens strategies for the patient with dry eye. Ocul Surf 2007; 5(4): 294-307. |
| [4] | Dumbleton K, Woods CA, Jones LW, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens 2013; 39(1): 93-9. |
| [5] | Pucker AD, Tichenor AA. A Review of contact lens dropout. Clin Optom 2020; 12: 85-94. |
| [6] | Sulley A, Young G, Hunt C, McCready S, Targett MT, Craven R. Retention rates in new contact lens wearers. Eye Contact Lens 2018; 44(1): S273-82. |
| [7] | Ramamoorthy P, Nichols JJ. Compliance factors associated with contact lens-related dry eye. Eye Contact Lens 2014; 40(1): 17-22. |
| [8] | Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf 2017; 15(3): 511-38. |
| [9] | The 2019 Study of the US Consumer Contact Lens Market. Princeton, NJ Multi-sponsor Surveys, Inc. 2020. |
| [10] | Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy report. Ocul Surf 2017; 15(3): 575-628. |
| [11] | FDA 510(k) Summary K200528. Bausch + Lomb (kalifilcon A) Soft (hydrophilic) Contact Lens. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf20/K200528.pdf. (Accessed on: 10th Sep 2022). |
| [12] | BAUSCH + LOMB launches Infuse SiHy daily disposable lens. Contact Lens Spectr 2020; 35(10): 58. |
| [13] | Okumura Y, Inomata T, Iwata N, et al. A review of dry eye questionnaires: Measuring patient-reported outcomes and health-related quality of life. Diagnostics 2020; 10(8): 559. |
| [14] | Wirth RJ, Edwards MC, Henderson M, Henderson T, Olivares G, Houts CR. Development of the contact lens user experience: CLUE scales. Optom Vis Sci 2016; 93(8): 801-8. |
| [15] | Nichols JJ, Mitchell GL, Nichols KK, Chalmers R, Begley C. The performance of the contact lens dry eye questionnaire as a screening survey for contact lens-related dry eye. Cornea 2002; 21(5): 469-75. |
| [16] | Dumbleton K, Caffery B, Dogru M, et al. The TFOS international workshop on contact lens discomfort: Report of the subcommittee on epidemiology. Invest Ophthalmol Vis Sci. 54(11): TFOS20-36. |
| [17] | Silicone Hydrogel Daily Disposable Symptom Tracker Quantitative Report. Boston, MA: Kadence International 2019. |
| [18] | Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017; 15(3): 276-83. |
| [19] | Stapleton F, Stretton S, Papas E, Skotnitsky C, Sweeney DF. Silicone hydrogel contact lenses and the ocular surface. Ocul Surf 2006; 4(1): 24-43. |
| [20] | Iskeleli G, Karakoç Y, Aydin O, Yetik H, Uslu H, Kizilkaya M. Comparison of tear-film osmolarity in different types of contact lenses. CLAO J 2002; 28(4): 174-6. |
| [21] | Saliman NH, Morgan PB, MacDonald AS, Maldonado-Codina C. Subclinical inflammation of the ocular surface in soft contact lens wear. Cornea 2020; 39(2): 146-54. |
| [22] | Efron N. Contact lens wear is intrinsically inflammatory. Clin Exp Optom 2017; 100(1): 3-19. |
| [23] | López-de la Rosa A, González-García MJ, Calonge M, Enríquez-de-Salamanca A. Tear inflammatory molecules in contact lens wearers: A literature review. Curr Med Chem 2020; 27(4): 523-48. |
| [24] | Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci 2009; 50(8): 3671-9. |
| [25] | Mathews PM, Karakus S, Agrawal D, Hindman HB, Ramulu PY, Akpek EK. Tear osmolarity and correlation with ocular surface parameters in patients with dry eye. Cornea 2017; 36(11): 1352-7. |
| [26] | Park J, Choi Y, Han G, et al. Evaluation of tear osmolarity measured by I-Pen osmolarity system in patients with dry eye. Sci Rep 2021; 11(1): 7726. |
| [27] | Bron AJ, Tiffany JM, Yokoi N, Gouveia SM. Using osmolarity to diagnose dry eye: A compartmental hypothesis and review of our assumptions. Adv Exp Med Biol 2002; 506(Pt B): 1087-95. |
| [28] | McMonnies CW. An examination of the relationship between ocular surface tear osmolarity compartments and epitheliopathy. Ocul Surf 2015; 13(2): 110-7. |
| [29] | King-Smith PE, Nichols JJ, Nichols KK, Fink BA, Braun RJ. Contributions of evaporation and other mechanisms to tear film thinning and break-up. Optom Vis Sci 2008; 85(8): 623-30. |
| [30] | Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: A literature review. Clin Ophthalmol 2014; 8: 1419-33. |
| [31] | Tonge S, Jones L, Goodall S, Tighe B. The ex vivo wettability of soft contact lenses. Curr Eye Res 2001; 23(1): 51-9. |
| [32] | Chen W, Zhang X, Li J, et al. Efficacy of osmoprotectants on prevention and treatment of murine dry eye. Invest Ophthalmol Vis Sci 2013; 54(9): 6287-97. |
| [33] | Aslan Bayhan S, Bayhan HA, Muhafız E, Bekdemir Ş, Gürdal C. Effects of osmoprotective eye drops on tear osmolarity in contact lens wearers. Can J Ophthalmol 2015; 50(4): 283-9. |
| [34] | Montani G. Intrasubject tear osmolarity changes with two different types of eyedrops. Optom Vis Sci 2013; 90(4): 372-7. |
| [35] | Guillon M, Maissa C, Ho S. Evaluation of the effects on conjunctival tissues of Optive eyedrops over one month usage. Cont Lens Anterior Eye 2010; 33(2): 93-9. |
| [36] | Hua X, Su Z, Deng R, Lin J, Li DQ, Pflugfelder SC. Effects of L-carnitine, erythritol and betaine on pro-inflammatory markers in primary human corneal epithelial cells exposed to hyperosmotic stress. Curr Eye Res 2015; 40(7): 657-67. |
| [37] | Corrales RM, Luo L, Chang EY, Pflugfelder SC. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea 2008; 27(5): 574-9. |
| [38] | VanDerMeid K, Millard K, Devolgado M, Reindel W, Rah M. Evaluation of erythritol and glycerin osmoprotection characteristics on an ocular surface cell line under hyperosmotic conditions. Poster presentation (Board 286 205286) at the American Academy of Optometry meeting, October 7-22, 2020 2020. Available from: https://aaopt.org/past-meeting-abstract-archives/?SortBy=&ArticleYear=2020&Title=&Abstract=&ArticleAuthor=VanDerMeid |
| [39] | Byrnes MG, Millard K, McGrath D, et al. Comparative analysis of the osmoprotective effects of a novel contact lens packaging solution on human corneal epithelial cells. Invest Ophthalmol Vis Sci 2021; 62: 672. |
| [40] | Goswamy S. Glycerine eye drops in keratopathy. Indian J Ophthalmol 1983; 31(4): 389-90. |
| [41] | Messmer EM. Osmoprotektion als therapeutisches Prinzip. Ophthalmologe 2007; 104(11): 987-90. |
| [42] | Bachman WG, Wilson G. Essential ions for maintenance of the corneal epithelial surface. Invest Ophthalmol Vis Sci 1985; 26(11): 1484-8. |
| [43] | Green K, MacKeen DL, Slagle T, Cheeks L. Tear potassium contributes to maintenance of corneal thickness. Ophthalmic Res 1992; 24(2): 99-102. |
| [44] | Ubels JL, Schotanus MP, Bardolph SL, Haarsma LD, Koetje LR, Louters JR. Inhibition of UV-B induced apoptosis in corneal epithelial cells by potassium channel modulators. Exp Eye Res 2010; 90(2): 216-22. |
| [45] | Hoteling AJ, Nichols WF, Harmon PS, et al. Characterization and quantitation of PVP content in a silicone hydrogel contact lens produced by dual-phase polymerization processing. J Biomed Mater Res B Appl Biomater 2018; 106(3): 1064-72. |
| [46] | Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson memorial lecture. Am J Ophthalmol 2011; 152(6): 900-909.e1. |
| [47] | Guzmán M, Miglio M, Keitelman I, et al. Transient tear hyperosmolarity disrupts the neuroimmune homeostasis of the ocular surface and facilitates dry eye onset. Immunology 2020; 161(2): 148-61. |
| [48] | Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul Surf 2013; 11(4): 246-58. |
| [49] | Fonn D. Targeting contact lens induced dryness and discomfort: What properties will make lenses more comfortable. Optom Vis Sci 2007; 84(4): 279-85. |