All published articles of this journal are available on ScienceDirect.
Vogt-Koyanagi-Harada-like Syndrome and Electronegative Electroretinogram after Nivolumab Therapy for Metastatic Cutaneous Melanoma: A Case Report
Abstract
Background:
Vogt-Koyanagi-Harada-like (VKH) syndrome or electronegative electroretinograms (ERG) have both been described before or after immunotherapy for metastatic cutaneous melanoma, but they have not been described occurring together.
Objective:
The objective of this study is to describe a case of VKH-like syndrome occurring simultaneously with an electronegative ERG after nivolumab therapy for metastatic cutaneous melanoma.
Methods:
Case report of a patient with ocular findings after nivolumab therapy for metastatic melanoma was reported. Multimodal imaging, including color fundus photography, optical coherence tomography (OCT), and both full-field and multifocal ERGs were used to describe the findings. Literature review was conducted with PubMed.
Results:
We reported a case of a patient with nivolumab-treated melanoma presenting with presumed VKH-like syndrome with panuveitis, choroidal depigmentation, and cutaneous vitiligo, as well as melanoma-associated retinopathy (MAR)-like electronegative ERG findings. Nivolumab was stopped and corticosteroid therapy was initiated. Although the patient’s visual acuity remained severely limited, her inflammation resolved, and the areas of choroidal depigmentation slowly decreased over years of subsequent follow-up.
Conclusion:
Vision loss accompanied by simultaneous VKH-like findings with choroidal vitiligo and a MAR-like electronegative ERG may develop after nivolumab therapy. The uveitic and vitiligo may improve with immunosuppressive therapy, but the vision loss and ERG findings may persist.
1. BACKGROUND
Immune checkpoint inhibitors (CI) confer survival benefits compared with conventional chemotherapy in metastatic melanoma. They activate T-lymphocytes against tumor cells by blocking the PD-1 or CTLA-4 receptors on T lymphocytes or the PDL-1 ligand on the tumor cells [1]. Despite their antitumor efficacy, CIs may lead to T-lymphocyte cross reaction with normal tissues, resulting in several autoimmune phenomena, especially against melanocytic cells such as skin, retinal pigment epithelium (RPE), and choroid [2]. One study suggested these autoimmune features are indicative of the treatment efficacy [3]. Several studies have reported various ocular side effects including anterior uveitis and a syndrome similar to Vogt-Koyanagi-Harada-syndrome (VKH), an autoimmune condition against ocular melanocytes characterized by panuveitis, serous retinal detachments, and choroidal vitiligo [4]. Another study noted cutaneous and choroidal vitiligo with combination CI therapy but lacked the other ocular inflammatory findings present in VKH [5].
As an independent phenomenon, patients with melanoma are at risk for developing melanoma associated retinopathy (MAR). An autoimmune retinopathy (AIR) targeting retinal bipolar cells, MAR can occur as a paraneoplastic syndrome or uncommonly in response to a CI [5]. When patients treated with CI for melanoma present with vision loss and uveitis, it can be difficult to distinguish whether the chorioretinopathy is a result of CI-associated inflammation or MAR. Here we report a unique case of the VKH-like syndrome with anterior uveitis, choroidal depigmentation, and cutaneous vitiligo triggered by nivolumab therapy, with concomitant electronegative electroretinogram (ERG), which may be secondary to MAR.
2. CASE PRESENTATION
A 63-year-old Hispanic female presented with approximately six months of right eye vision loss. Her past medical history was notable for diabetes mellitus type II, hypercholesterolemia, and a history of foot melanoma resected one year prior who was since started on nivolumab. Six months after initiation of nivolumab, she simultaneously developed hypopigmented skin lesions and perceived visual decline. Two months after the start of vision loss and eight months after nivolumab initiation, an outside optometrist recorded her vision at 20/70 OU. Four months later, when she presented to our clinic, her visual acuity (VA) was counting fingers at one foot in the right eye and 20/40 in the left eye with normal intraocular pressures and without a relative afferent pupillary defect. Ishihara color plates were 0/12 right eye and 12/12 left eye. Slit lamp exam was notable for 2+ cell in the right eye and 1+ vitreous cell. Fundus exam demonstrated scattered RPE changes, focal hypopigmented lesions, microaneurysms, dot-blot hemorrhages, and normal appearing optic nerves. She was diagnosed with VKH-uveitis from nivolumab immunotherapy, hospitalized for intravenous corticosteroids, and nivolumab was stopped.
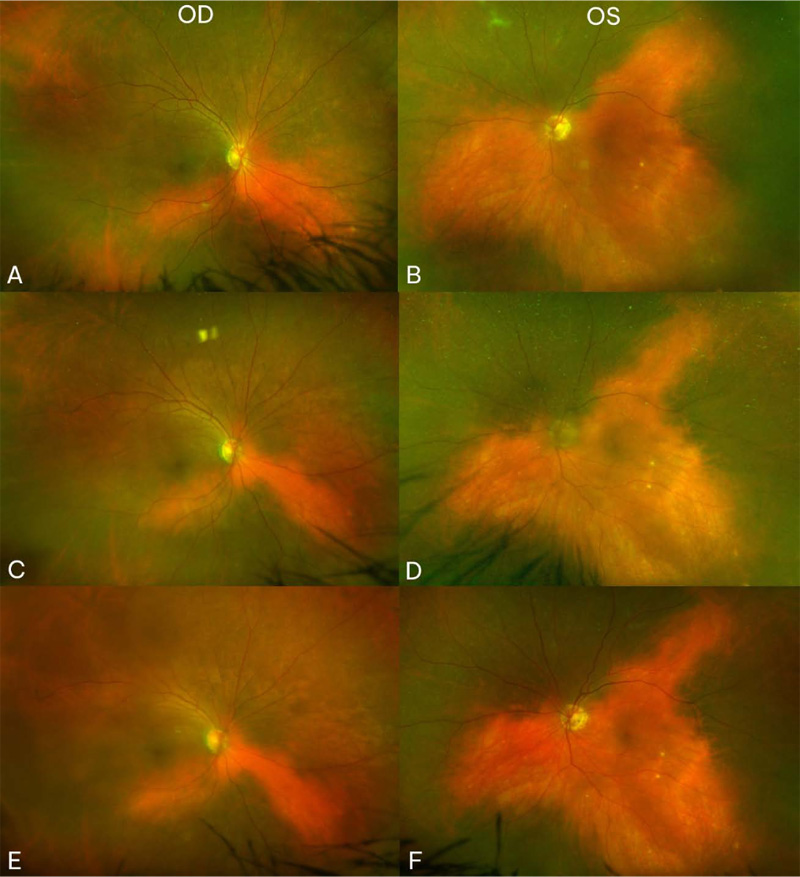
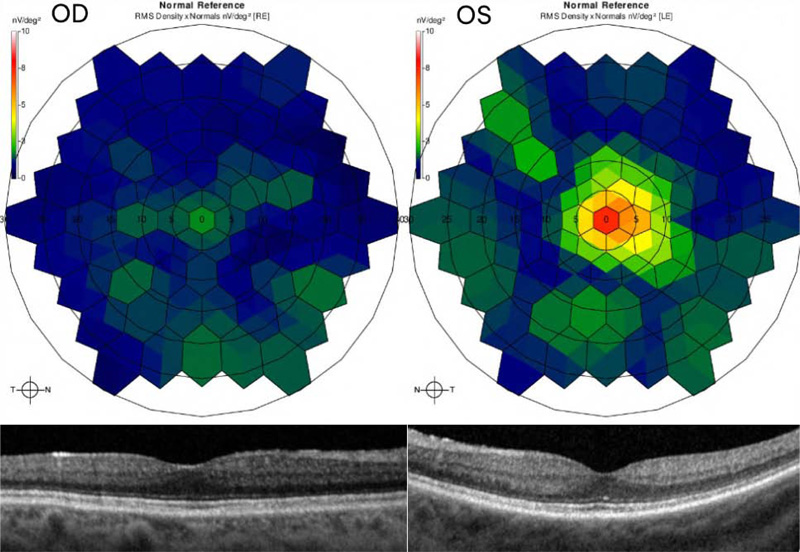
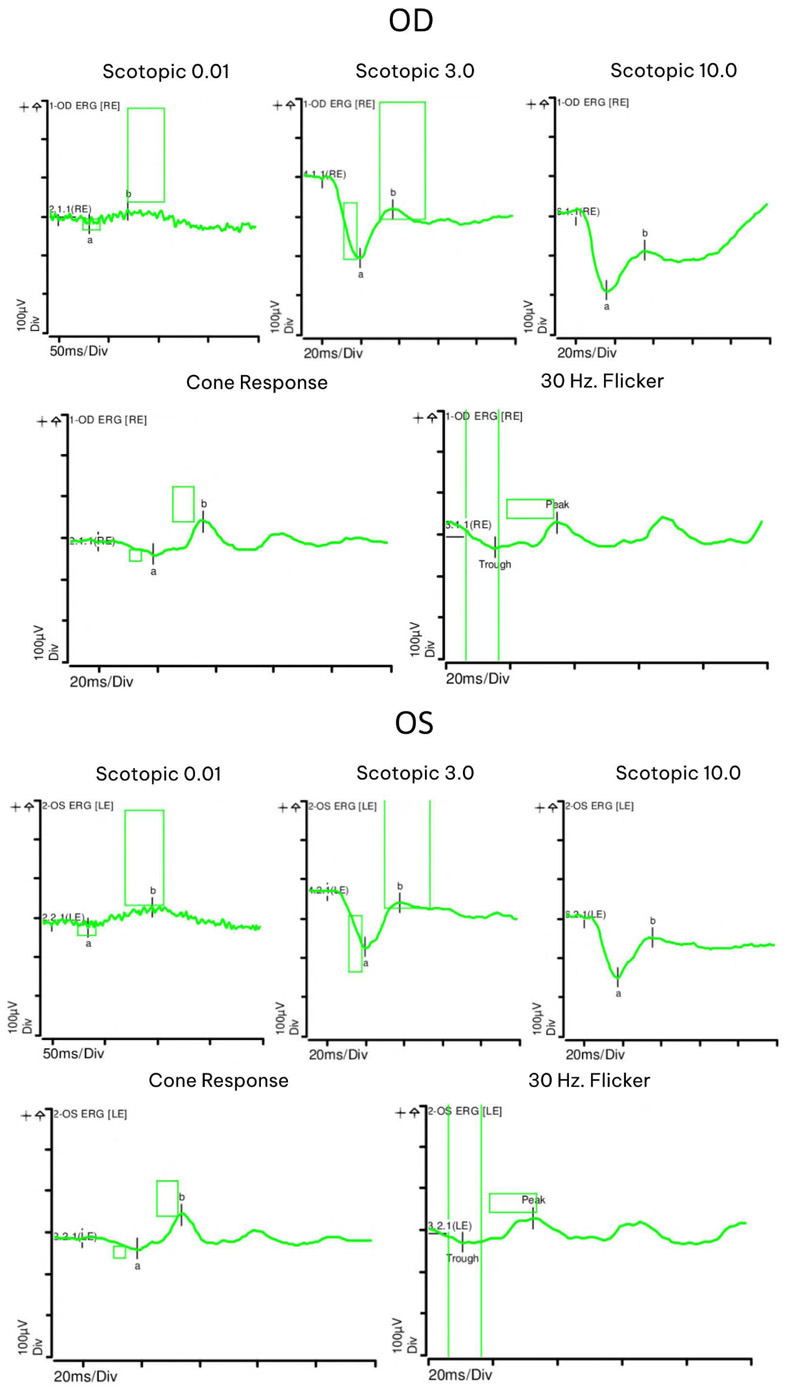
At seven month follow-up, her vision remained stable and her uveitis resolved. Fundus exam displayed choroidal vitiligo that slightly improved over three years (Fig. 1). Optical coherence tomography (OCT) demonstrated intact retinal layers in both eyes (Fig. 2). Retinal nerve fiber layer (RNFL) thicknesses and macular ganglion cell complex (GCC) thicknesses on OCT were normal bilaterally. Multifocal ERG showed decreased macular function worse in the right eye (Fig. 2). Magnetic resonance imaging (MRI) of brain and orbits was notable only for chronic microvascular angiopathy. Goldmann visual fields demonstrated a cecocentral scotoma in the right eye. Full field ERG disclosed abnormal rod and cone function with electronegative waveforms in both eyes (Fig. 3). Her vision, exam findings, and ERGs remained stable over three additional years of follow up. The patient was not interested in testing for anti-retinal antibodies.
3. DISCUSSION
Ocular side effects with CIs, including uveitis, have been well-documented [4], and autoimmune retinopathies both arising and improving from treatment with CIs have been described [5-8]. However, simultaneous findings of electro-negative ERG and CI-related uveitis with choroidal and cutaneous vitiligo have not been reported to our knowledge. Our case provides evidence that a VKH-like uveitic reaction and MAR-like electronegative ERG can occur in patients with metastatic melanoma treated with checkpoint inhibitor immunotherapy.
A higher rate of ocular side effects have been reported with ipilimumab, and more frequently with nivolumab than with the PD-1/PD-L1 inhibitors [4]. Moreover, combination nivolumab/ ipilimumab therapy has been suggested to increase immuno-genicity [6]. While a wide variety of ocular findings have been described, anterior uveitis and a VKH-like spectrum have been noted repeatedly [9-14]. Cutaneous vitiligo has been a noted side effect of both CI [15, 16] and melanoma [17]. Our patient developed cutaneous vitiligo and asymmetric uveitis with bilateral choroidal vitiligo after CI therapy, suggesting an autoimmune reaction to the CI. While choroidal hypopigmentation is a classic finding in chronic idiopathic VKH, fewer reports have been noted in CI-associated panuveitis [4, 12, 17].
Since electronegative ERG is a known finding in MAR, it is possible that our patient may have also had MAR. The heightened immune response promoted by CI has been purported to result in anti-retinal antibody creation. The antibodies in MAR cross react with bipolar cells, resulting in characteristic electronegative ERG waveforms and preservation of photoreceptors [18]. Vision changes from MAR are variable but often improve with treatment [19], although they may take decades to improve [20]. Additional findings such as vitreous cell and vascular attenuation have been noted in a subset of patients [20], but our patient demonstrated anterior uveitis and severe unilateral VA loss with corresponding asymmetric multifocal ERG changes in the setting of symmetric full-field ERG findings consistent with MAR. The spectrum of AIR findings after CI therapy for melanoma was noted to be quite variable in one series, including onset of visual symptoms varying from one week up to two years after CI initiation [6]. Our patient’s vision only slightly improved over the three years of follow up.
One case with CI-related uveitis without VKH-like findings did note progressive fundus depigmentation bilaterally as well as cutaneous vitiligo and poliosis, as in our case, but with preserved visual acuities [21, 22]. Another patient with MAR developed depigmented fundus lesions with pigment clumping during pembrolizumab therapy [23]. The authors suggested that the fundus changes could be secondary to an unrestrained immune system after CI treatment for MAR. Both cutaneous and choroidal melanocytes derive from the neural crest during embryogenesis, resulting in occasional simultaneous vitiligo such as in VKH [23].
One reported patient with metastatic melanoma developed decreased VA, decreased b-wave ERG amplitude and cutaneous vitiligo; subsequent ipilimumab therapy resulted in further decreased vision and increased vitiligo size [7]. Similarly in our patient, nivolumab treatment likely resulted in autoimmunity to the melanoma, retina, and choroidal and cutaneous melanocytes. However, it is also possible that the uveitic and ERG findings preceded institution of nivolumab therapy, but clinical and diagnostic testing had not been performed during that window. Additionally, the patient developed symptoms only after initiating nivolumab. However, the patient declined retinal antibody testing that may have supported an autoimmune phenomenon. Visual complaints and impaired visual acuity may result with both drug-induced uveitis and autoimmune retinopathies; therefore, it is important to conduct appropriate imaging and functional testing to determine the etiology of vision impairment in this population and to guide therapy.
CONCLUSION
In this report, we present the first, to our knowledge, case of simultaneous vitiliginous panuveitis and electronegative ERGs in a patient treated with immunotherapy for metastatic melanoma . Although cessation of nivolumab and corticosteroid treatment resulted in resolution of uveitis and gradual improvement in choroidal vitiligo in our patient, her vision changes and ERG findings persisted over the following three years. Given the multifaceted, permanent ocular injury that can result from CI treatment, it may be prudent to recommend routine ocular screening of patients who are started on CI treatment. Routine electrophysiologic testing in addition to clinical imaging of these patients may provide more insight into the incidence of this phenomenon and guide therapeutic interventions.
LIST OF ABBREVIATIONS
| VKH | = Vogt-Koyanagi-Harada-like |
| ERG | = Electronegative Electroretinograms |
| OCT | = Optical Coherence Tomography |
AUTHORS' CONTRIBUTIONS
All authors collected the study data, wrote, revised, and approved the manuscript. Cameron Pole, Erin Su, Niranjana Kesavamoorthy, and Hossein Ameri helped in writing the manuscript, and Christian Sanfilippo and Kimberly Gokoffski edited, reviewed, and revised the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTI-CIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
The patient gave written consent to be anonymously published.
AVAILABILITY OF DATA AND MATERIAL
The authors confirm that the data supporting the findings of this study are available within the manuscript.
STANDARDS OF REPORTING
CARE guidelines were followed in this study.
FUNDING
This study was funded by Research to Prevent Blindness, New York, NY, Unrestricted grant to the Department of Ophthalmology of the University of Southern California, USA.
CONFLICT OF INTEREST
The authors report no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


