Acanthamoeba and Pseudomonas Co-infection after Cataract Surgery
Abstract
Background:
We report a case of endophthalmitis secondary to a co-infection with Acanthamoeba and Pseudomonas aeruginosa post-cataract surgery in a peripheral hospital in Lebanon.
Case Presentation:
The patient presented to our department after having a phacoemulsification and intraocular lens implantation at a peripheral hospital.
Case Study:
Proper management and interventions were made to salvage the eye.
Conclusion:
To our knowledge, the literature has no prior cases of endophthalmitis with such a co-infection.
1. INTRODUCTION
Infectious endophthalmitis is a threatening ocular pathology that can occur spontaneously or following an ocular trauma or intraocular surgery. Pseudomonas aeruginosa (P. aeruginosa) is an aerobic gram-negative microorganism that can be present and survive in diverse aqueous solutions, surgical equipment and disinfectants, which makes it a potent source of nosocomial infections [1]. Exogenous endo- phthalmitis due to Pseudomonas infection, occurring after an ocular surgery or trauma, frequently have poor outcome despite maximal therapeutic interventions [2].
For more than 2 decades, it has been found that gram-positive microorganisms are the most common causative agents of postoperative endophthalmitis, with rare cases of gram-negative bacteria isolated [3, 4].
Ocular Acanthamoeba infection typically presents with severe treatment-resistant keratitis, secondary to direct corneal invasion, usually affecting contact lens wearers, or presenting after ocular trauma or ocular exposition to contaminatedwaters. The rare involvement of the posterior ocular segment is usually the consequence of long-standing keratitis in immuno- suppressed individuals [5,6], but as mentioned, Acanthamoeba endophthalmitis following ocular surgery is very rare [7]. In a recent case series of 116 eyes with exogenous endophthalmitis at a Colombian reference center, Acanthamoeba spp. was not isolated from any of them [4]. This demonstrates how rare it is that the etiology of intraocular infection is by this parasite, but, on the other hand, an outbreak of four cases caused by Amoebas has been published [7].
According to our knowledge, the case herein reported of intraocular co-infection (Acanthamoeba spp. and Pseudomonas aeruginosa) following cataract surgery is the first one published in scientific literature.
2. CASE PRESENTATION
The case conformed to the tenets of the Declaration of Helsinki, where informed consent was obtained from the patient prior to all interventions and reporting.
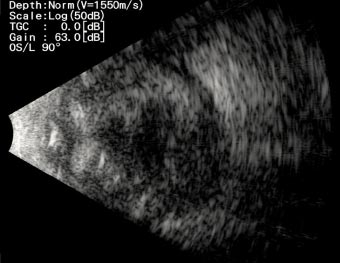
A 76-years-old male patient presented to our department for a second opinion, after presenting severe left eye (OS) pain, photophobia and loss of vision, 13 days after undergoing phacoemulsification and intraocular lens implantation at a peripheral hospital in Lebanon. He reported hypertension and hypothyroidism under proper management and has had phacoemulsification and intraocular lens implantation in the right eye (OD) 4 years before.The patient reported pain, redness and a drop in vision OS had developed in the first postop day. He was started on moxifloxacin, and steroid eye drops at a frequency of 5 times per day in another facility. The patient stated that his symptoms worsened, for which topical dorzolamide, timolol and fusidic acid gel were added to the regimen. He received 4 intravitreal injections of vancomycin and ceftazidime, apparently without any intravitreal tap done for microbiological studies. Uncorrected visual acuity (UCVA) was 20/60 in the OD, and hand motion (HM) in the OS. The globe OS was soft on palpation. Dilated slit lamp exam showed a non-injected conjunctiva OD, clear sclera, clear cornea with no ulcers or defects, deep and quiet anterior chamber, a posterior chamber intraocular lens, clear vitreous, normal retinal vasculature, an optic nerve with clear borders with no cupping and a clear macula. Slit lamp exam OS showed a red and injected conjunctiva, mucopurulent discharge arising from the main incision site supero-temporally, a 9 mm central corneal white opacity with irregular borders reaching deep stromal layers, and fibrinous reaction in the anterior chamber associated with a hypopyon which grading couldn’t be identified due to severe corneal opacification. A photo slit lamp was taken and shown in Fig. (1). Fundus in OS could not be visualized, for which an ocular ultrasound was performed, showing multiple dispersed vitreal opacities, flat retina and choroid, and no retained lens fragments or foreign bodies (Fig. 2). All the clinical findings were suggestive of acute postoperative endophthalmitis. No information could be retrieved regarding preoperative measurements of disinfection and the intraoperative course of the recent surgery.
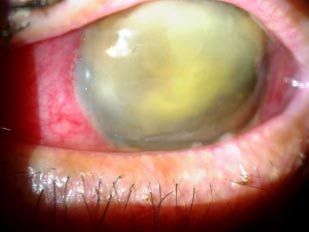
A corneal swab from the corneal ulcer was taken for culture in addition to a swab from the discharge at the incision site. The patient was immediately started on moxifloxacin eye drops every 2 hours, tobramycin ocular ointment 3 times per day, dorzolamide and timolol drops twice per day, and fortified vancomycin eye drops every 2 hours, all of which were to be applied OS. The patient had received his fore-mentioned intravitreal injections shortly prior to presentation (the last one being 24 hours prior to presentation), thus, further injections were postponed until culture results were obtained. Preliminary microbiological results showed gram-negative staining with the presence of unidentified cysts for which the treatment was adjusted accordingly; moxifloxacin every 2 hours, fortified vancomycin every 4 hours, fuscidic acid gel twice a day, and itraconazole 100mg 1 tab twice a day assuming that the cysts observed are due to Acanthamoeba.
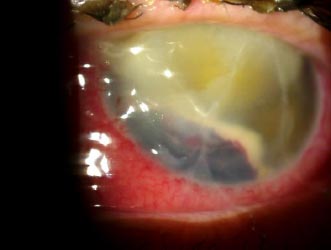
Upon follow-up on a daily basis, OS examination showed a grade 2 hyphema in addition to an increase in the purulent discharge from the incision site (Fig. 3). Over the course of 3 days, the OS slit lamp exam was stable except for a decrease in discharge at the incision site; the same treatment regimen was continued.
Four days after the culture samples were taken, official culture results returned positive for Pseudomonas Aeruginosa with the presence of Acanthamoeba cysts. According to the sensitivity profile (sensitive to ciprofloxacin with the lowest minimum inhibitory concentration being 0.12 μg/mL), the treatment regimen was adjusted where ciprofloxacin eye drop was added every 2 hours. Propamidine isethionate 0.1% (Brolene) every 1.5 hours was added to the treatment regimen. Still, it was started 3 days later due to its unavailability in the Lebanese market and the need to import it from London. Topical antiamoebic drugs like polyhexamethylene biguanide (PHMB) 0.04%, chlorhexidine 0.02 to 0.04%, voriconazole 1% or intracameral and intravitreal voriconazole were also considered to be started, but it was impossible to provide these medications due to their unavailability in locoregional pharmacies and difficulty obtaining them from abroad within a short timeframe.
The patient was examined serially while on the same treatment, with the slit lamp examination, OS showing stable ocular findings with no worsening nor improvement. Repeat ocular ultrasound showed persistent vitreal opacities.
The patient underwent an intravitreal injection of fortified antibiotics (ceftazidime and vancomycin) one-week post presentation. An attempt of vitreal and anterior chamber tap using needle aspiration prior to injections was done. Still, it was deemed unsuccessful due to the high viscosity of the discharge present in both chambers. One-week post-injection, the patient complained of severe headaches over the left frontal and temporal areas. Slit lamp examination showed a completely white anterior chamber and a diffusely injected conjunctiva OS with a persistent 9mm central corneal ulcer; a soft globe was noted on palpation. An ocular ultrasound was done and showed an increase in the vitreal opacities, a decrease in the axial diameter OS, and a retinal flap was noted, suggestive of retinal detachment. A brain and orbit MRI confirmed a smaller left globe with evidence of retinal detachment, extensive scleral, vitreal swelling, and corneal swelling, all of which are compatible with pan-endophthalmitis. No retro-orbital abscess was seen. After 3 weeks of incubation, cultures revealed no fungal growth and the patient was maintained on the previously mentioned ocular and systematic treatment. A decision of enucleation was discussed with the patient in the setting of deteriorating eye status despite all the therapeutic regimens being used. Enucleation OS was done and multiple vitreal and anterior chamber samples were obtained for culture all of which showed no bacterial, parasitic or fungal growth.
3. DISCUSSION
First and foremost, it is important to highlight the socio-economic crisis that Lebanon is facing. In 2022, The World Bank illustrated that Lebanon is almost three years into an economic and financial crisis that is among the worst in the world has seen [8]. In conjugation with the COVID-19 pandemic, this crisis caused the pharmaceutical and medical sectors to suffer from working with minimal capabilities leading to a drastic drop in medical and sanitary product availability and quality. With the increase in impoverished citizens, patients started seeking affordable medical care (medical visits, imaging, procedures etc.) in rural and underdeveloped healthcare facilities with minimal to no quality control imposed by the State. This discussion will demonstrate the interventions that were ideally required to be performed in such a case, however, limitations that prevented optimal outcomes are also discussed. [9-12]
The patient presented with an advanced stage of endophthalmitis (VA < hand motion) with a white cornea. Guidelines for the management of endophthalmitis advise for vitrectomy with debulking and IOL removal in cases of VA of light perception or worse [13-15]. In this situation, vitrectomy is indicated but it wasn’t feasible because trans-corneal visibility in our case was very poor where the patient had already presented late and keratoplasty was not possible due to the unavailability of donor corneal graft, high procedure cost and the inability to perform a keratoplasty with an active infectious etiology. Moreover, all surgical devices used during the procedure must have been promptly disposed of, manifesting once again escalating costs.
The patient had a VA of HM, and treatment with intravitreal antibiotics was recommended. However, upon presentation, the patient had already received 4 intravitreal injections, the last being in the past 24 hours. The decision to reinject was cautiously delayed avoiding the risk of retinal toxicity.
Upon presentation, the diagnosis of endophthalmitis was clear to us, and ideally, it should be confirmed with a vitreal tap and culture. However, a vitreal and anterior chamber tap was deemed unsuccessful due to the high viscosity of the fluids. The negative culture results post-enucleation probably suggest the eradication of the infection existing beforehand due to the topical, intra-vitreal and systemic antibiotics given. The patient’s deteriorating ocular condition, despite maximal medical therapy necessitated the need for an enucleation as the final medical intervention.
The patient’s postoperative history showed no evidence of contact with potentially contaminated water or misuse of prescribed antibiotic drops. The patient was immunocompetent and not taking any systemic medication that could compromise his immune system. Hence, the possibility of a perioperative infection source is highly suspected, especially since the perioperative and intraoperative disinfection course is unknown to the patient and us. In the medical literature, several modes of transmission of postoperative Pseudomonas have been described. Ramappa et al. report 11 cases of acute post-cataract endophthalmitis with Pseudomonas traced down to contaminated hydrophilic intraocular lens solution [9]. In another study, contamination was shown to be due to the internal fluid system of the phacoemulsifier [10, 11]. Kenchappa et al. describe cases in which the source of infection was narrowed down to the phaco probe [11]. Finally, Eifrig et al suggest that the main source of infection was a contaminated air-conditioning system [12].
As for endophthalmitis due to Acanthamoeba, there are only few cases reported worldwide in which the route of transmission was not postulated.
In line with other post-cataract endophthalmitis due to Pseudomonas species cases, broad spectrum topical and systemic antibiotic therapy with vancomycin and ciprofloxacin was started immediately [9, 12, 16,17]. Unfortunately, anti-Acanthamoeba therapy was delayed; it was started 3 days after the initial visualization of cysts due to the unavailability of the drug in the country. After visualization of the Acanthmoeba cysts, PCR testing should have ultimately been performed as a more accurate test, but it was not cost-effective to be done as the patient was already started on anti-Acanthamoeba eye drops and any PCR done will not change the management [18].
This approach highlights again the financial difficulties and restraints that we are facing on daily basis in order to provide optimal treatment for such difficult cases.
As described in the literature, acute-onset of endo- phthalmitis caused by Pseudomonas is often aggressive, resistant to treatment and associated with poor visual outcomes despite prompt treatment. In a published study where 12 of 52 cases had endophthalmitis caused by Pseudomonas, 8 of these 12 had no light perception and 7 patients were either eviscerated or enucleated as a final outcome [19]. In another study by Eifrig et al, 64% of the patients with Pseudomonas concomitant endophthalmitis were enucleated or eviscerated and 35% had a final visual acuity of less than 5/200 [12].
Unfortunately, the potential source of infection could not be traced to the initial peripheral hospital and cultures from the operative theater could not be obtained. The economic crisis the country is suffering from has affected the quality of patient care and hygiene measures taken by health care parties amidst the shortage of medical equipment and pharmaceutical products. The absence of public health guidelines and surveillance protocols is a nidus for the emergence of breaches in disinfecting measures especially in rural hospitals. The Ministry of public health and national medical authorities should implement strict regulations for disinfection in hospitals and medical practice institutions. Besides, there should be a system for the detection and tracing of infections acquired from health institutions for the purpose of prevention and limitation of malpractice measures.
CONCLUSION
In our report, we described the case of a 76-year-old immunocompetent male patient who presented after cataract surgery with aggressive pan-endophthalmitis due to two culprit organisms: Pseudomonas and Acanthamoeba. This co-infection has, to our knowledge, never been reported in the literature. Multiple difficulties and obstacles were faced with providing the optimal medical management for this patient due to the high cost of medical interventions (with the patient having no insurance or social security plan) and drug unavailability in the Lebanese pharmaceutical market. The economic crisis in Lebanon and consequent decline in medical sanitary and sterilization standards in some peripheral medical centers should not be dealt with at ease to prevent further threatening and resilient infections as the one fore mentioned.
LIST OF ABBREVIATIONS
| HM | = Hand Motion |
| PHMB | = Polyhexamethylene Biguanide |
| UCVA | = Uncorrected Visual Acuity |
AVAILABILITY OF DATA AND MATERIALS
This case report’s discussion and data were based on the research databases and in specifically the references of the respected article, which are enlisted below.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Fadi Maalouf, who performed the enucleation for the patient.


