All published articles of this journal are available on ScienceDirect.
MGrx - A Novel Multi-modal Thermal Device for Treating Moderate to Severe Meibomian Gland Dysfunction and Dry Eye
Abstract
Background:
MGD (meibomian gland dysfunction) is a chronic cause of dry eyes. Thermal expression of the meibomian glands, along with massage and debridement, is an effective treatment for MGD.
Objective:
We describe a multi-modal thermal device (MGrx) to manage meibomian gland dysfunction (MGD). We observed the efficacy and safety of the MGrx to manage MGD in one 15-minute in-office session.
Methods:
We enrolled 37 patients in a prospective, open-label trial of the novel MGrx. Patients were enrolled with a Standard Patient Evaluation for Eye Dryness (SPEED) score > 12 or a Tear Breakup Time (TBUT) of < 6 seconds in at least one eye. After screening for eligibility, one 15-minute MGrx treatment was provided to each patient. The patient assessment consisted of a SPEED score, TBUT, and a Meibomian gland score (MGS) obtained pre-treatment and at a follow-up visit 30 days after the treatment.
Results:
Dry eye symptoms improved in the patient population, as measured by SPEED score, MGS, and TBUT, by 40%, 341%, and 145%, respectively (p<0.05). No adverse reactions were noted among the patients.
Conclusion:
A single 15-minute MGrx treatment was effective at significantly improving dry eye symptoms secondary to MGD in adult patients, as measured by SPEED score. Additionally, a single MGrx treatment improved meibomian gland function and all measures of MGD in the adult patients treated. Given the relatively low risk and efficient delivery of the MGrx treatment, a single MGrx treatment should be considered as a first-line treatment for MGD.
1. INTRODUCTION
One of the leading causes of dry eyes is dysfunction of the meibomian glands, also known as meibomian gland dysfunction (MGD) [1, 2]. Although MGD can include cases of either hypersecretion or hyposecretion of meibum, most cases of MGD involve blockage and obstruction of the meibomian glands [2], with associated decreased secretion of meibum. Meibum is a key component to maintain the lipid tear layer in contact with the surface of the eye, and its absence leads to excessive evaporation of tears from the eye surface, resulting in dry eye symptoms [2].
There is no consensus on the optimal means of treating dry eye due to MGD [3]. The ultimate goal is to restore meibum secretion and improve dry eye symptoms. To achieve this, a diverse set of treatments exists, including massage, heat treatment, and the use of antibiotics and/or anti-inflammatories [3]. The protocol for managing MGD in these patients usually begins with proper eyelid hygiene, for e.g., the patient massaging the eyelids, typically after applying heat or warm compresses, and then debriding the eyelid. If this is ineffective, artificial lubricants, antibiotics, anti-inflammatories, lipid diet supplements, cyclosporine and, more rarely, surgery can be indicated [3].
Since patient compliance with eyelid hygiene can be challenging, several heat-based devices have emerged in the market that provide thermal lid massage (MiBoFlo Thermoflo®, Mibo Medical, Inc.) or automated heat and pressure (LipiFlow®, Johnson & Johnson, Inc.) to offer the benefits of thermal therapy, but with a more convenient treatment schedule [3]. Numerous studies have shown that a single heat-based treatment can effectively improve MGD in patients with dry eye [4-7]. A novel, multi-modal thermal device (MGrx, OcuSci®, Inc.) has been developed that enables thermal lid debridement, thermal lid massage, and thermal gland expression. We have described our initial experience and observations using the MGrx to treat patients with diagnosed dry eye (Fig. 1).
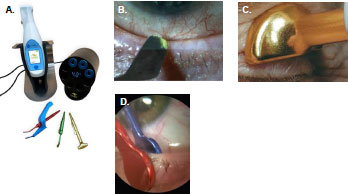
2. MATERIALS AND METHODS
The study included 37 adult patients with MGD and dry eye. All participants were enrolled in an open-label, active treatment with the MGrx, where neither providers nor patients were blinded. All participants signed a written consent form after a thorough informed consent process. This study was performed adhering to the Declaration of Helsinki, with regards to the treatment of human subjects.
2.1. Inclusion Criteria
To qualify for the study, patients were required to have symptoms of dry eyes and MGD as determined by patient history and a Standard Patient Evaluation for Eye Dryness (SPEED) score of > 12 or a Tear Breakup Time (TBUT) of < 6 seconds in at least one eye. Additional inclusion criteria included age >18 and willingness to comply with the study protocol and follow-up schedule.
2.2. Exclusion Criteria
Exclusion criteria included inability to consent or follow-up, MGD grade 3 atrophy, eyelid deformity or eyelid movement disorder, active or history of corneal/conjunctival pathology, active ocular allergy, use of cyclosporine eye drops, patients wearing contact lenses, and the use of warfarin or other anticoagulants. The amount of atrophy in the lower eyelid was graded on a 0-3 scale, where 0=no atrophy, 1=1-33% atrophy, 2=34-66% atrophy, and 3=66% or more atrophy.
2.3. Patient Evaluation
A comprehensive dry eye evaluation was conducted at the preliminary visit, including a slit lamp evaluation of the tear lake (TBUT, corneal staining evaluation, and SPEED score). After the first 11 patients were enrolled, we added the collection of a meibomian gland score (MGS) for the subsequent patients for each eye. The eyelids were inspected using a Meibomian Gland Evaluator (MBE, TearScience®). The MGS was calculated by assessing the number of glands producing meibum multiplied by the quality of meibum secreted as follows: clear = 3, cloudy = 2, inspissated = 1. A follow-up examination was performed thirty days post-treatment and included the same components as the initial exam, i.e., SPEED score, TBUT, and MGS.
2.4. MGrx and Treatment Protocol
The MGrx has three modes of action, including thermal debridement, thermal massage, and a thermal expression mode. The hand-held device is reusable and enables the use of three different instrument attachments to deliver the three modes of treatment. The instruments and modes can be changed with the push of a button. Instruments can be autoclaved and are reusable. There are no disposables required to use the MGrx. The MGrx weighs 6 ounces and is designed ergonomically for use on the eyelids. The unit is rechargeable and one charge allows multiple patient treatments. Each treatment session lasts 10-15 minutes.
Therapy with the device was performed in an office setting by a trained technician (thermal massage mode only) or an optometrist. Topical anesthetic drops were applied to the patient’s eyes prior to both thermal debridement and thermal expression treatments, using the device in a standard manner. Each treatment session was performed with the patient seated and in the supine position. Eyelids were treated one at a time. Treatment involved 1-2 minutes (per eye) of thermal debridement performed at the lid margin, followed by 2 minutes of thermal massage therapy to each closed eyelid and then 1-2 minutes (per eye) of thermal meibomian gland expression on all four eyelids. After treatment, post-treatment MGS was calculated for each eye.
| Variable | N |
Pre-treatment (mean ± SD) |
Post-treatment (mean ± SD) |
|---|---|---|---|
| Age | 36 | 57 ± 14 | |
| Gender | M= 12, F = 24 | NA | |
| SPEED score | 36 | 14.4 ± 5.9 | 8.6 ± 5.6 |
| TBUT OD | 36 | 2.7 ± 2 | 6.4 ± 2.3 |
| TBUT OS | 36 | 2.7 ± 2.1 | 6.8 ± 2.1 |
| MGS OD | 26 | 4.9 ± 4 | 23 ± 7 |
| MGS OS | 26 | 5.5 ± 4.9 | 22.7 ± 7.7 |
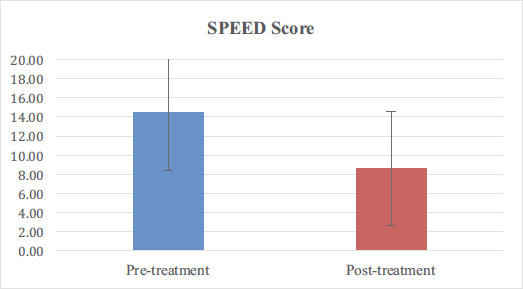

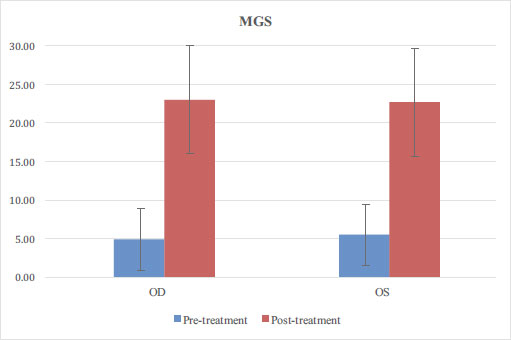
2.5. Statistical Analysis
The primary endpoints included a change in SPEED score, a change in TBUT, and a change in MGS. Descriptive statistics was utilized for demographics, SPEED score, TBUT, and MGS. Conventional 2-sided t-testing was performed for continuous variables, with a p < 0.05 being statistically significant. This analysis was performed with Google Sheets.
3. RESULTS
A total of 36 patients completed the study protocol (one enrolled patient was lost to follow-up). Of these, 12 were men and 24 were women with an average age of 57 ± 14. Baseline demographics and patient variables are further detailed in Table 1. Statistically significant improvement (p < 0.05) was noted in SPEED score (14.4 ± 5.9 before, 8.6 ± 5.6 after; Fig. 2) and TBUT (OD 2.7 ± 2 before, 6.4 ± 2.3 after; OS 2.7 ± 2.1 before, 6.8 ± 2.1 after; Fig. 3). A total of 26 patients had an MGS recorded for both eyes, and improvement was also statistically significant: OD 4.9±4 before, 23±7 after; OS 5.5±4.9 before, 22.7±7.7 after (Fig. 4). No adverse reactions were noted in any patients.
4. DISCUSSION
In the current study, 36 patients were treated with the MGrx to manage MGD. This was done in an open-labeled, unblinded setting. A statistically significant improvement in SPEED score, TBUT, and MGS for both eyes was noted after the treatment. No adverse reactions were noted in this small cohort. These initial data are very promising and suggest that MGrx should be considered as a first-line treatment for MGD.
Dry eye disease (DED) is a multifactorial disease of the tears and ocular surface that afflicts hundreds of millions of patients around the world [8]. In the U.S. alone, 40 million people are estimated to suffer from, or to be predisposed to, this debilitating condition [9]. DED is mostly age-related [2, 8], but it can also be triggered by refractive or cataract surgery [2]. In addition, preexisting DED significantly increases the risk of prolonged or severe post-operative signs and symptoms of dry eye after refractive or cataract surgery [2]. Refractive and cataract surgery patients have high visual expectations after surgery, and increasingly sophisticated intraocular lens and corneal ablation designs heighten the importance of good ocular surface health. The success of refractive and cataract surgeries is therefore, in many cases, fundamentally dependent on effectively addressing preexisting or iatrogenic DED. The most common form of DED is evaporative, which is mainly due to MGD [2].
The current management paradigm for DED due to MGD is evolving [3, 9, 10]. Patients are initially managed with local eyelid hygiene, including eyelid massage and meibum debridement. A critical part of eyelid hygiene is warming the eyelids and consequently the meibum, which allows for better removal of meibum that is thickened [11, 12]. If the eyelid’s hygiene is insufficient in resolving dry eye symptoms, a number of other therapies exist. These include antibiotics, especially doxycycline, anti-inflammatory eye drops, such as cyclosporine, lubricating eye drops, dietary interventions, and, rarely, surgery [2, 9-12].
It is well-established that eyelid hygiene is a key component of the management of MGD [3, 10]. In addition, twice daily eyelid massage after warming has recently been shown to be an effective treatment for MGD [11, 12]; however, adherence to such a treatment schedule can be challenging, and thus, a more durable, less intense solution is desirable.
Our outcome data are consistent with several recent trials of thermal eyelid treatment devices. In Hagen’s trial [7] that compared LipiFlow® to doxycycline, the LipiFlow® device led to a 5.5-point improvement in SPEED score, while our results showed a 5.8-point improvement. In the same trial, LipiFlow® showed a 2.2-second improvement in TBUT, while the MGrx resulted in a 2.7-second improvement. The comparator arm in Hagen’s trial was 3 months of oral doxycycline, which, although effective, is cumbersome and comes with several possible side effects. The MGrx does not have any of the potential toxicities associated with doxycycline therapy, such as photosensitivity, esophagitis, and drug-drug interactions. Furthermore, the MGrx does not involve any issues associated with compliance, as daily antibiotic therapy for three months can be a challenge, even for the most compliant patients.
An additional device that has been studied for the treatment of MGD is the MGDRx® Eyebag® [13-18]. In the trial by Bilkhu et al., patients required twice-daily therapy for a total of two weeks [18]. Although improvement in multiple dry eye parameters was observed, this is a challenging treatment schedule for many patients. Furthermore, patients who fared best with MGDRx® Eyebag® continued treatments multiple times for a month, with a range of 1-8 treatments being noted in the trial [18]. This multi-treatment schedule is in a single, 15-minute thermal treatment with the MGrx.
Similarly, the Blephasteam® device [19] presents a challenging treatment schedule. Patients are required to use the Blephasteam® device for 10-15 minutes two times a day and continue treatment for three weeks. In the trial conducted by del Castillo et al., this was the treatment schedule utilized [19]. Furthermore, the primary outcome in this trial was a single endpoint of patient symptom score [19]. Although improvements were noted, this was not as robust a study design or as quantitative as that of our trial, utilizing SPEED score, TBUT, and MGS in a standardized manner, both before and after therapy to assess response.
Finally, compared to a number of other available therapies for DED due to MGD, the MGrx has distinct advantages. The existing treatments for MGD have been summarized in a 2015 review article by Thode [20]. Compared to selenium-based shampoos or others, the MGrx does not involve the risk of ocular or skin allergy. Likewise, omega-3 fatty acids show efficacy in treating this condition; however, patient adherence to a daily regimen of supplements remains an issue; however, a single session with the MGrx could be advantageous. Similarly, terpinen-4-ol from tea tree oil can be effective, but only when Demodex mites are an issue, thus limiting the applicability of this treatment. Treatment with either topical macrolides, such as azithromycin or a systemic macrolide/doxycycline, carries inherent drug-related side effects and toxicities, along with possible patient compliance issues. Cyclosporine and/or steroid eye drops also carry toxicity and compliance issues, as well as the risk of cataracts and glaucoma [20].
There are several advantages of a rapid, in-office treatment with the MGrx. First, this treatment can be delivered in an outpatient setting, with minimal setup and training. Second, this is a low-risk treatment, with no obvious post-treatment adverse effects for the patient. Third, adherence to therapy is likely to be much improved with a treatment reported to be comfortable and even pleasurable by patients, which may be needed periodically, versus daily self-performed therapy.
Most instruments and devices used for treating MGD [e.g., LipiFlow®, Systane® iLux2®, TearCare®, MiBoFlo Thermoflo®, and intense pulsed light (IPL) devices] require actuators (typically $250-$300 per treatment) and/or multiple treatments, which adds significant cost to the complete treatment protocol [21]. The MGrx requires no actuators and is designed to improve symptoms after one treatment. The MGrx reusable instruments can be autoclaved or sterilized with alcohol wipes. These features substantially reduce the cost of the MGrx to both providers and patients. In addition, the MGrx is lightweight, portable, and easy to move from room to room.
MGD treatments are not covered by insurance and can be prohibitively expensive for some patients. Currently, patients are charged roughly $400 to $1,200 per course of treatment. For IPL therapy, each treatment is typically around $300 and patients need at least three treatments one month apart for effective results [22, 23]. This therapy is further limited, as African American patients cannot be treated with the device due to the risk of skin burn [22]. Furthermore, treatments can be painful, thus limiting the patient population who are willing to undergo further IPL treatment [22].
Since the MGrx has no actuators or additional per-treatment costs, clinicians can offer this thermal MGD treatment at a price that meets their market and is not dictated by a fixed variable cost. Our investigators reported charging patients $250-450 per treatment during the study. In addition, the device is lightweight and portable, allowing the clinician to take the device from room to room. This portability could be a tremendous improvement in workflow within practice, as a treatment room is typically dedicated to a much larger IPL or thermal pulsed therapy device.
While the current study lays the groundwork for future in-depth studies, there are several notable limitations. First, this was an uncontrolled, open-label study. As such, we do not know how the MGrx would compare with an active control, such as another thermal device, or a medical therapy being used in parallel. Additionally, the optimal study design to assess the MGrx would be a double-blinded trial, testing it head-to-head with another thermal device. Such a trial may prove technically challenging and costly to conduct, as blinding the operator of each device may be difficult.
Secondly, as a retrospective study, there are inherent flaws associated with this type of analysis. There can be unrecognized confounders in the analysis that overstate the results. Additionally, patient selection may influence the observed outcomes. A randomized study population or a prospective trial may yield different results.
Finally, we have had a complete dataset for only 26 of the enrolled patients and partial data for 36; thus, larger cohort studies will be required to more comprehensively understand the risks and benefits of the MGrx. The lack of MGS in 10 of the patients may lead to a bias in the MGS scores and make the results appear better than if scores were available for the entire cohort.
CONCLUSION
In this initial study, the MGrx showed statistically significant improvement in dry eye symptoms, as demonstrated by SPEED score measurements, strongly suggesting it to be an effective treatment for improving signs and symptoms of dry eye disease in patients with MGD. The cost-effective design of the system, requiring no single-use actuators and a single-session treatment protocol, may enable more patients with dry eye symptoms to be treated for MGD. Future studies will help solidify the role of MGrx in the management of MGD.
LIST OF ABBREVIATIONS
| MGD | = Meibomian Gland Dysfunction |
| SPEED | = Standard Patient Evaluation for Eye Dryness |
| TBUT | = Tear Breakup Time |
| MGS | = Meibomian Gland Score |
| DED | = Dry Eye Disease |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the institutional ethics committee of OcuSci, Inc., Del Mar, CA.
HUMAN AND ANIMAL RIGHTS
No animals were used in the study. This study was performed in accordance with the Declaration of Helsinki, with regard to the treatment of human subjects.
AVAILABILITY OF DATA AND MATERIAL
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The data that support the findings of this study are available from the corresponding author, MHNM, on special request.
CONFLICT OF INTEREST
The authors confirm that there is no conflict of interest related to the manuscript.
ACKNOWLEDGEMENTS
Drs. McMurren, Kling, and Fasciani performed the clinical research study at their respective practices. Dr. Nymark-McMahon, an independent consultant, was hired to write and edit the manuscript. The MGrx Multi-modal Thermal Device was provided for the study by OcuSci, Inc., Del Mar, CA. No additional funding was provided to the clinicians or patients.


