RESEARCH ARTICLE
Pseudophakic Cystoid Macular Edema Associated with Extrafoveal Vitreoretinal Traction
Michael R Martinez 1, Avinoam Ophir*, 1, 2
Article Information
Identifiers and Pagination:
Year: 2011Volume: 5
First Page: 35
Last Page: 41
Publisher ID: TOOPHTJ-5-35
DOI: 10.2174/1874364101105010035
Article History:
Received Date: 28/10/2010Revision Received Date: 21/1/2011
Acceptance Date: 22/1/2011
Electronic publication date: 12/05/2011
Collection year: 2011

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Purpose:
To describe an association between extrafoveal vitreoretinal traction and intractable chronic pseudophakic cystoid macular edema (CME) by the use of optical coherence tomography (OCT).
Methods:
In a retrospective case series study, charts and OCT findings of patients who had postoperative recalcitrant pseudophakic CME for at least 6 months and vitreoretinal traction membranes were analyzed. Excluded were eyes that either had another vitreoretinopathy that could affect the analysis or had undergone an intravitreal intervention.
Results:
Three eyes (three patients) with macular edema following uneventful cataract surgery were detected to be associated with multifocal extrafoveal vitreoretinal traction sites in each. Retinal edema that was underlying each of the traction sites in all eyes was in continuum in at least one site per eye with the central macular edema, thus manifesting as diffuse macular edema.
Conclusion:
Chronic pseudophakic macular edema may be related to extrafoveal vitreoretinal traction.
INTRODUCTION
Cystoid macular edema (CME) is the most common retinal cause of vision impairment after uncomplicated cataract surgery [1]. Pseudophakic CME develops angiographically in up to 20% to 30% of patients after uneventful extracapsular cataract extraction/phacoemulsification, and it usually takes six to eight weeks to develop [2-4]. In most patients, pseudophakic CME resolved spontaneously, with 50% to 75% of patients achieving improved vision within six months. However, clinically significant CME, defined as a Snellen visual acuity of 20/40 or worse, accompanied by cystoid spaces or retinal edema, has been reported to occur in 2.35% of these cases [5].
Most investigators agree that inflammatory mediators (e.g., prostaglandins) and vascular endothelial growth factor (VEGF) may be associated with disruption of the blood-retinal barrier, with resultant fluid accumulation in the perifoveal area [6, 7]. Vitreous traction at the anterior segment structures and ocular hypotony are other factors that have been related to the occurrence of pseudophakic CME [8]. Prompt treatment of the disorder is required since irreversible macular changes might occur if macular edema is present for several months [9]. Several pharmacologic agents are commonly used to treat this condition, including topical, sub-Tenon's, intravitreal and systemic corticosteroids, as well as oral and topical nonsteroidal anti-inflammatory agents (NSAIDs), but some patients do not respond to these medications [10]. Recently, antiangiogenic agents such as bevacizumab (Avastin) have been implicated in the treatment of pseudophakic CME [11]. However, the benefit of the anti-VEGF agents for pseudophakic CME, which are widely applied for most forms of macular edema is somewhat controversial [11, 12]. In a study that presented a positive beneficial outcome of intravitreal bevacizumab [11], it was so in 72.2% (26/ 31) of the eyes, but the benefit was, by and large, temporary, whereas the remaining 27.8% did not respond to that therapy. As for surgery, Harbour et al. (n = 24) and Pendergast and associates (n = 23) reported on the outcome of pars plana vitrectomy (PPV) in eyes with recalcitrant pseudophakic CME [13, 14]. In both studies, diminution of the macular edema was substantial, but while there was marked improvement in Snellen best-corrected visual acuity (BCVA) in the former study, it was mild in the latter.
Similarly, refractory chronic macular edema may occur in a variety of other pathologic conditions, such as diabetic retinopathy, retinal vein occlusion (RVO) and posterior uveitis [15-17]. Using optical coherence tomography (OCT), which provides an “optical biopsy” of the macula and the posterior pole in a histological level of resolution, enabled the establishment of vitreofoveal traction as another documented cause of macular edema in these ocular diseases [18]. Recently, vitreous traction membranes that are located extrafoveally, either retinal or at the optic nerve head (ONH; i.e. vitreopapillary traction), have also been found to be associated with macular edema in eyes with diabetic retinopathy using spectral-domain (SD)-OCT [19], as well as in non-diabetic eyes using time-domain (TD)-OCT [20]. The retinal edema that underlined the extrafoveal traction site in these studies was commonly in a continuum with the macular edema, thus presenting as diffuse macular edema.
This study presents (for the first time, to the best of our knowledge; search via Entrez PubMed), a small series of patients in whom chronic macular edema of pseudophakic origin was found to be associated with extrafoveal vitreoretinal traction in each.
MATERIALS AND METHODOLOGY
In a retrospective case series study we analyzed the charts and TD-OCT scans (OCT 2000, Humphrey Zeiss inc., San Leandro, CA, USA), and later the SD-OCT scans (SD-OCT 1000, Topcon Corp., Tokyo, Japan), of patients who had chronic (> 6 months) macular edema and vitreoretinal traction following cataract extraction and intraocular lens implantation. Clinical examination included BCVA and slit-lamp and fundus examinations. OCT examination of all eyes was thereafter performed through a dilated pupil by one of two trained examiners.
Using the TD-OCT, evaluation of macular edema was routinely initiated by using the Automatic 6-radial lines program directed to the fixation point and focused on the central macula. This was followed using the Line group program, which the examiner controls manually. Scans were taken at various angles and lengths (a shorter scan increases resolution) to search for a vitreous traction site at both the area centralis (between the vascular arcades) away from the macula and the ONH site. Evidence of traction required vitreous adherence to the retina or ONH associated with, a) tissue elevation and deformity at the traction site, i.e., the shape of the inner retina at the exact site of traction changed its angle and thus was typically thicker than that of the adjoining edematous retinal tissue and, b) the posterior hyaloid or vitreous strand terminated or changed its angle at that site. Vitreous traction at one site (“unifocal”) away from the edematous central macula was designated as “extrafoveal traction”, either at the area centralis (“extrafoveal vitreoretinal” traction) or at the ONH (“vitreopapillary” traction). When there were two or more traction sites, traction was designated as “multifocal”. Vitreous “adherence” (without traction) related to those eyes in which the attachment of the vitreous was not associated with any vitreoretinal or vitreopapillary deformities of these tissues at that site. When an extrafoveal vitreous traction was detected, that site was examined to determine whether or not its underlying retinal edema (or subretinal fluid) was in continuum with the central macular edema.
Using the SD-OCT, 3-dimensional data sets centered on the fovea (6mm X 6mm) were obtained for each patient. As a rule, 3-D data sets were also centered on the ONH in association with the central macula and were obtained using a raster scan program of 8.2 mm (horizontal) x 3 mm (vertical) x 1.7 mm (axial). Volumetric rendering of the data set was performed using image-processing software within the SD-OCT for 3-D image reconstruction. The SD-OCT characteristics are described elsewhere [21]. Data, including the B-mode and 3-D mode, were evaluated later by examining the recorded videos.
Cystoid spaces at the foveal region, as detected by the OCT, were defined as intraretinal round hyporeflective lacunae with well-defined boundaries and hyper-reflective septa separating the cystoid-like cavities. Diffuse macular edema was identified by OCT as an ill-defined and widespread, hyporeflective, increased retinal thickness that often attains the appearance of sponge-like cavities [22, 23]. The 6-mm Early Treatment Diabetic Retinopathy Study (ETDRS) macular maps and the OCT false-colors maps provided quantitative and qualitative information on the thickness of the retinal tissue at the site in question, respectively, and were quantitatively compared with the normal controls. We used our normative TD-OCT (n = 12) and SD-OCT (n = 50) database of normal macular thickness in age-matched patients as controls. Based on these, macular thickness using TD-OCT or SD-OCT was considered thickened when its central sub-field (CST) exceeded 200 µm (TD-OCT) or 250 µm (SD-OCT), similar to data previously reported [24, 25]. The fovea could be designated as edematous when cystoid spaces were located at its site, even if the tissue was not abnormally thickened, due to the fact that it could be thin due to atrophy or lamellar hole formation.
Patients were included in the study if they had both chronic macular edema (> 6 months) that appeared following cataract extraction and was related to vitreoretinal traction membranes. Exclusion criteria covered eyes that had: 1) another retinopathy that could affect the data analysis; 2) chronic uveitis; 3) vitreoretinal adherence without signs of retinal traction or vitreous traction without macular edema; 4) undergone vitreoretinal surgery; 5) been treated by intravitreal administration of medication(s), or 6) eyes in which the OCT scans were of too low a quality for a proper diagnosis and measurements.
Research adhered to the tenets of the Declaration of Helsinki, and the approval of the Institutional Review Board was obtained.
RESULTS
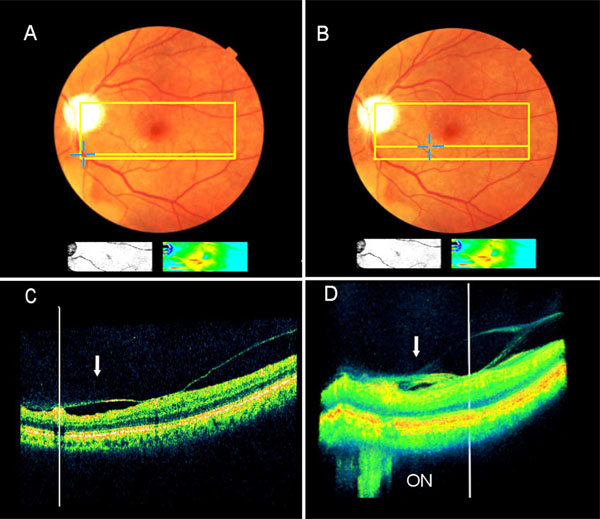
Three patients, aged 51-78 years old, with chronic pseudophakic CME in one eye, in which any other potential clinical cause for the edema was not detected, were diagnosed using OCT to have extrafoveal multifocal vitreoretinal traction sites in each eye. Patients’ characteristics and OCT findings are summarized in Table 1. Both the B-mode video clips of the SD-OCT and their 3-D image reconstructions enabled fast and unambiguous detection of extrafoveal traction membranes in eyes No. 1 & 2 (Figs. 1, 2). The retinal edema that underlined each of the vitreoretinal traction sites in Eye No. 1, including the edema that was associated with the vitreopapillary traction, was in continuum with the central macular edema, thus attaining a pattern of diffuse macular edema. This observation could be depicted from the macular maps and the 3-D image reconstructions of the SD-OCT (Fig. 1 and supplementary video clip 1). Eye No. 2 had Irvine-Gass syndrome with a swollen disc at the early postoperative period. Four years later, using SD-OCT, the perifoveal area was mildly thickened, and a pseudohole at the central macula with adjacent ERM was apparent. The retinal edema that underlined one of the two detectable traction sites was in continuum with the central macular edema in that eye (Fig. 2).
Data of Patients with Pseudophakic Cystoid Macular Edema
| Patient | A/G/E | BCVA | Central Sub-Field Thickness (in Microns) | Site of Extrafoveal Traction | Comments |
|---|---|---|---|---|---|
| 1 | 89/F/LE | 20/80 | 340 | Three sites: 1-2) 2 sites infero-nasal to fovea,3) at the vitreopapillary site | |
| 2 | 64/M/LE | 20/60 | 285 | Two sites: 1) Inferior to the optic nerve head,2) Inferonasal to fovea | Pseudohole |
| 3 | 53/M/RE | 20/60 | 370 | Two sites: 1) At the papillomacular bundle, 2) superotemporal to the fovea |
A=Age, G=Gender, E=Eye; BCVA=Best-corrected visual acuity; M=Male, F=Female; RE=Right eye; L=Left eye.
The third eye (No. 3) was examined by TD-OCT (OCT-2000) and presented with diffuse macular edema accompanied by cystoid spaces. Vitreoretinal traction membranes were identified at two sites, one parafoveally at the papillomacular bundle (PMB) zone and the second temporally to the fovea (Fig. 3; a 3-D image reconstruction is not available in the TD-OCT). The centrally fixated Automatic 6-radial lines program of the OCT-2000 enabled the diagnosis of extrafoveal vitreous traction, which was located parafoveally at the PMB site. A small area of subretinal fluid underlined this traction site. The second traction site in this eye was detected by the Line group program, but not by the Automatic 6-radial lines program. The retinal edema that underlined each of the two detected traction sites was in direct communication with the edema at the central macula. This observation could be depicted from the OCT B-mode and the macular map (Fig. 3C, D).
DISCUSSION
The study presents three eyes with refractory pseudophakic diffuse macular edema that was associated with multifocal extrafoveal vitreoretinal traction in each. The retinal edema that was underlying at least one traction site per eye was in continuum with the macula, manifesting in each as diffuse macular edema.
Several studies on PPV for treating recalcitrant pseudophakic CME have been published [13, 14, 26]. Harbour et al. reported on 24 consecutive patients who underwent PPV for chronic pseudophakic CME that failed to respond medically [13]. The surgery resulted in improved visual acuity in all eyes from a mean visual acuity of 20/190 preoperatively to 20/52 postoperatively, with a mean improvement of 4.7 Snellen lines. Peyman et al. reported on the beneficial effect of PPV and internal limiting membrane peeling, which took place in two eyes with recalcitrant pseudophakic CME 11-22 months after cataract surgery [26].
By contrast, Pendergast et al. reported the results of PPV for intractable chronic pseudophakic CME in 23 consecutive eyes with no apparent vitreoretinal disturbance. Surgery resulted in resolution of the CME in all cases, though with improved visual acuity in only some of the eyes [14]. This relatively low functional benefit could be related to the macular edema's having been present for a mean of 20 months (range, 3-110 months) prior to surgery. The suggestion of that possibility is based on earlier studies on PPV for diabetic macular edema (DME) [27, 28], which suggest that a delay in performing surgery may partly explain the permanently reduced vision in spite of marked structural improvement [27]. Other authors who found PPV for DME of benefit attributed it in part to the removal of growth factors associated with macular edema from the vitreous site [29]. However, the studies on PPV for pseudophakic CME did not mention or rule out an existence of extrafoveal vitreous traction, and the stated cause of surgery's success or failure of the surgery was often ambiguous. The presence of extrafoveal vitreous traction in the current study may be relevant to that issue.
The centrally fixated Automatic 6-radial lines program of the TD-OCT (OCT-2000) enabled unequivocal diagnosis of one site of vitreous traction in eye No. 3, which was located parafoveally at the PMB site (Fig. 3). Only the Line group program enabled the detection of the second traction site in this eye. As previously described, detection of extrafoveal traction can often be made only with the Line group or the Raster line programs [30]. This is due to the fact that the length of each scan in the Automatic 6-radial lines program is 5.9 mm, the scans are separated from each other by a 300 arc and program's output is limited to the 6 lines that are scanned. Furthermore, the length of the arc between two adjacent radial lines at their farthest point from the center is >1.5mm (2πR/ 12 = 2 X 3.14 X 3 / 12). Therefore, using the Line scan or the Raster line programs to search for an extrafoveal site is likely to require hundreds of scan lines in order to cover every point at the area centralis. As a consequence, many extrafoveal traction sites may be overlooked due to omission of vitreous adherence sites that are typically small, because of progressive patient fatigue in this time-consuming search, and because of progressive corneal epithelial drying that could result in poor scan quality.
In contrast, two eyes (No. 1 & 2) were plainly diagnosed to have multifocal extrafoveal traction membranes by the SD-OCT. With a scanning time of 3.7 seconds, the SD-OCT detected extrafoveal traction sites much faster than the TD-OCT did, and is also expected to have a higher rate of accuracy than the TD-OCT does [31]. That is, since the SD-OCT screens every point at the scanned vitreous membranes and retinal surface, acquiring an entire A-scan simultaneously, its B-mode has 40,000 A-scans per second (vs 400 A-scans/second in the TD-OCT), and it promises image acquisition at 50 to 100 times the TD-OCT speed with equal or improved spatial resolution. In addition, the improved 6µm axial resolution of the SD-OCT over the TD-OCT (10-15µm) allows better identification of morphologic details in the retina and the vitreoretinal interface, as previously described [32, 33]. The 3-D image reconstruction of the SD-OCT may provide more detailed information than its B-mode can because it reveals a real image, both horizontally and vertically, of the whole vitreoretinal area under study with better spatial perception of the vitreoretinal interface. Such an image is depicted, for example, in Fig. (1) and supplementary video clip No. 1.
Except for the potential for consideration of PPV in these eyes, detection of extrafoveal traction should also be important if a therapeutic agent like bevacizumab or triamcinolone acetonide (kenalog) is planned intravitreally, since doing so can result in worsening of traction and subsequent traction retinal detachment [34].
Limitations of the study refer to its retrospective design and small series. However, the study raises the possibility that recalcitrant pseudophakic CME or macular edema may be associated with extrafoveal vitreoretinal traction. The SD-OCT detected the traction sites much faster and with a higher rate of confidence than the TD-OCT could. Further studies and a larger cohort are required to validate whether early vitrectomy is the treatment of choice in these situations.
VIDEO CLIP NO. 1 (PATIENT NO. 1)
This article also contain supplementary material (3-D movie clip) ans it can be viewed at publisher’s website.
The 3-D movie shows 2 continuous vitreoretinal traction sites. Each of the traction sites is underlain by retinal edema that is in continuum with the central macula, thus presenting as diffuse macular edema (see also Fig. 1).