All published articles of this journal are available on ScienceDirect.
Functional Roles of Electrogenic Sodium Bicarbonate Cotransporter NBCe1 in Ocular Tissues
Abstract
Electrogenic Na+-HCO3- cotransporter NBCe1 is expressed in several tissues such as kidney, eye, and brain, where it may mediate distinct biological processes. In particular, NBCe1 in renal proximal tubules is essential for the regulation of systemic acid/base balance. On the other hand, NBCe1 in eye may be indispensable for the maintenance of tissue homeostasis. Consistent with this view, homozygous mutations in NBCe1 cause severe proximal renal tubular acidosis associated with ocular abnormalities such as band keratopathy, glaucoma, and cataract. The widespread expression of NBCe1 in eye suggests that the inactivation of NBCe1 per se may be responsible for the occurrence of these ocular abnormalities. In this review, we discuss about physiological and pathological roles of NBCe1 in eye.
INTRODUCTION
The presence of electrogenic Na+-HCO3-cotransporter NBCe1 was first demonstrated in 1983 by the electro-physiological experiment in isolated renal proximal tubules from salamander Ambystoma tigrinum [1]. In view of the large capacity of renal proximal tubules to absorb bicarbonate, NBCe1 was considered to play an important role in maintaining acid-base balance of extracellular fluid. Romero and colleagues first succeeded in NBCe1 cloning [2]. NBCe1 has two major spliced variants, the kidney type cotransporter NBCe1-A and the pancreas type cotransporter NBCe1-B as shown in Fig. (1). They originate from the same gene SLC4A4, and differ only at the N-terminus [3]. NBCe1-A encodes 1035 amino acids and predicts a protein of 116 kDa. NBCe1-B has unique N-terminal 85 amino acids replacing the first 41 amino acids of NBCe1-A. This NBCe1-B encodes 1079 amino acids and predicts a protein of 120 kDa. Another variant NBCe1-C (rb2NBC1) was also cloned from rat brain [4]. This variant has 61 unique C-terminal amino acids, the result of a 97-bp deletion and frame shift near the end of the open-reading. The encoded rat protein is 1094 amino acids and predicts a protein of about 130 kDa. This C-terminal NBCe1 variant has been also identified in human (GenBank accession number EF531618.1). However, the physiological significance of brain type variant remains to be clarified. The structure of NBCe1 is predicted based on the structure of anion exchanger AE1 [5]. NBCe1-B is widely expressed in several tissues such as pancreas [6,7], eye [8,9], colon [6] or brain [4,6]. While NBCe1-B may mediate bicarbonate secretion from pancreatic duct cells in pancreas [10], it may also mediate the maintenance of tissue homeostasis in the ocular tissues [8,9]. On the other hand, NBCe1-A is thought to represent a major pathway for basolateral bicarbonate exit from renal proximal tubules [10]. In support of this view, homozygous mutations in NBCe1 have been shown to cause severe proximal renal tubular acidosis (pRTA) associated with ocular abnormalities [11]. Because of the widespread expression of NBCe1 in several ocular tissues, the inactivation of NBCe1 seems to be directly responsible for ocular abnormalities [8]. Transport stoichiometry of NBCe1 seems to be either 1Na+:3HCO3- or 1Na+:2HCO3- [12-15]. Difference of the number of transported ions designates ‘electrogenic’. While NBCe1-A favors the efflux transport mode from cells with 1Na+:3HCO3- stoichiometry in renal proximal tubules, NBCe1-B favors the influx transport mode into cells in corneal endothelium or pancreatic ducts with 1Na+:2HCO3-stoichiometry. However, the stoichiometry of NBCe1 can change depending on the cell type in which it is expressed or other factors such as intracellular Ca2+ concentrations [16-18].
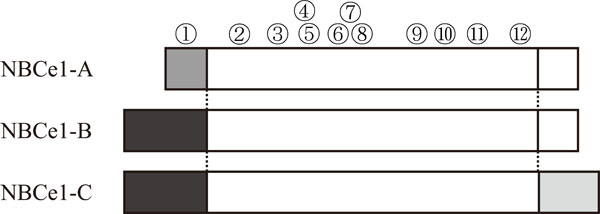
Structures of NBCe1 variants and pRTA-related mutations. Different patterns in the squares indicate unique amino acids sequences. While NBCe1-A has a unique N-terminal, NBCe1-C has a unique C-terminal. Numbers in circles indicate pRTA-related NBCe1 mutations corresponding to Q29X (1), R298S (2), S427L (3), T485S (4), G486R (5), R510H (6), W516X (7), L522P (8), N721TxfsX29 (9), A799V (10), R881C (11), and S982NfsX4 (12). Q29X is an NBCe1-A specific mutation, leaving NBCe1-B and NBCe1-C intact. S982NfsX4 creates a flame-shift mutation in NBCe1-A and NBCe1-B, but may suppress the translation of NBCe1-C. The remaining mutations lie in the common region of NBCe1 variants.
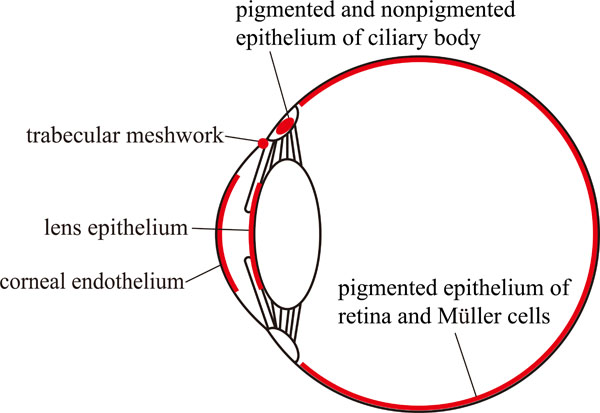
Major sites of NBCe1 expression in eye.
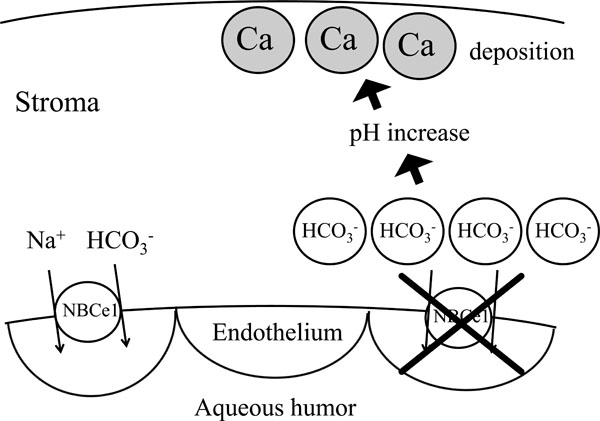
Proposed mechanism of band keratopathy by NBCe1 mutations. Inactivation of NBCe1 may increase the concentration of bicarbonate in corneal stroma, which may facilitate the calcium deposition.
MUTATIONS OF NBCE1 AND PATHOGENESIS
Igarashi and colleagues first identified that homozygous mutations in NBCe1 cause isolated severe pRTA and ocular abnormalities [11]. Up to now twelve cases of NBCe1 mutations have been reported [15,19-25]. They were eight missense mutations, two nonsense mutations, and two flame-shift mutations. Most of these patients with homozygous NBCe1 mutations have severe metabolic acidosis (serum bicarbonate is usually less than 13 mEq/l), stunted growth, and ocular abnormalities such as band keratopathy, glaucoma, and cataract. Some of them also presented with migraine as summarized in Table 1.
Phenotypes and Functional Properties of NBCe1 Mutations
| R298S | Q29X | S427L | T485S | G486R | R510H | W516X | L522P | N721TxfsX29 | A799V | R881C | S982NfsX4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | F | F | F | M | F | F | F | M | M | F | F | F |
| Number of patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| Country | Japan | Japan | USA | USA | Brazil | Japan | Taiwan | USA | USA | Turkey | UAE | Belgium |
| Blood HCO3- (mEq/l) (normal value: 24) | 8 | 9.4 | 11 | 13 | 3 | 5.5 | 10 | NA | 13.2 | 6.3 | 10.6 | 14.8 /17.3 |
| Band keratopathy | + | - | + | + | + | + | + | + | + | + | + | + |
| Glaucoma | + | + | + | - | - | + | + | + | + | + | + | + |
| Cataract | + | - | + | + | + | + | + | + | + | + | + | + |
| Migraine | - | - | NA | - | - | + | - | + | + | - | + | + |
| Proper membrane expression in mammalian cells | - | - | - | + | + | - | - | - | - | + | - | - |
| Activity in HEK293 cells | 39% | NA | NA | 30% | 42% | 6% | NA | 0% | NA | 32% | 4% | 3% |
Several expression systems have been used to analyze the functional impacts of NBCe1 mutations. For example, Xenopus oocytes are used for the evaluation of the electrophysiologic properties of NBCe1 [15,26]. However, the surface expression in oocytes cannot predict the trafficking behaviors of NBCe1 in the polarized cells, and polarized Madin-Darby canine kidney (MDCK) cells seem to be more suitable for the evaluation of NBCe1 trafficking abnormalities [21]. ECV304 and HEK293 cells are used for the evaluation of the transport activities of NBCe1 in mammalian cells [11,27]. C6 glioma cells, which retain several properties of astrocytes but lack the endogenous NBCe1 activity [28] are also used to evaluate the NBCe1 functions in astrocytes. Multiple experimental approaches to clarify the possible disease mechanism by NBCe1 mutations revealed that several mutations have loss of function (R298H, T485S, G486R and A799V). Other mutations show the trafficking abnormalities in mammalian cells (R298S, S427L, R510H, L522P, R881C and S982NfsX4). pRTA caused by NBCe1 mutations is always inherited by an autosomal recessive manner. However, we identified that the heterozygous NBCe1 S982NfsX4 mutation causes migraine and normal tension glaucoma without pRTA in an autosomal dominant manner. Although the S982NfsX4 mutant has the normal function as wild type in Xenopus oocytes, it shows trafficking defect that results in cytosolic retention in mammalian cells such as HEK293, C6 glioma and MDCK cells. Because S982NfsX4 and wild type NBCe1 form hetero-oligomer as reported [27], we speculated that the dominant negative effect by S982NfsX4 mutation might be responsible for migraine and glaucoma in the heterozygous family members [22].
EXPRESSION AND PHYSIOLOGICAL ROLE OF NBCE1 IN EYE
The expressions and physiological functions of NBCe1 have been demonstrated in several ocular tissues as shown in Fig. (2). For example, NBCe1 is thought to be expressed in the corneal endothelial cells. There is one study reporting the NBCe1 expression in rat corneal epithelial cells in addition to endothelial cells [9]. Bicarbonate flow out of cornea into aqueous humor is thought to be essential for the maintenance of corneal transparency [29]. Several reports suggested that NBCe1 is expressed only in the basolateral membrane of bovine and human corneal endothelial cells and to transport sodium and bicarbonate into aqueous humor [30-32]. Another group reported that NBCe1 is expressed in both bovine apical and basolateral membranes of endothelium [33]. On the other hand, NBCe1 specific siRNA knockdown in cultured bovine corneal endothelial cells decreased by 80-90% expression of the cotransporter and significantly reduced basolateral HCO3- permeability [34]. These data suggest that the basolateral NBCe1 may have a more important role in the corneal endothelium. Partial knockdown of NBCe1 in vivo in rabbit failed to affect the corneal thickness, however 25% NBCe1 knockdown induced higher corneal swelling relative to control eyes [35]. By contrast, near-complete inactivation of NBCe1 in vivo caused corneal edema and opacity [25], consistent with the essential role of NBCe1 in the maintenance of corneal transparency.
The expression of NBCe1 was found in both apical and basolateral membranes in lens epithelium of human and rat [8,9]. In the human lens epithelium cell lines, HLE cells, the transport activity of sodium bicarbonate cotransport was detected. Because this activity was largely suppressed by the specific hammerhead ribozyme against NBCe1, it might reflect the NBCe1 activity [8]. The lens is a tissue without vascular supply, and simple diffusion of nutrients may be insufficient to support its metabolic consumption [36]. One study indeed reported that the cultured lens epithelium can actively transport fluid from the anterior to the posterior side against a hydrostatic pressure [37]. Although the functions and importance of NBCe1 in lens epithelium still remain unclear, the vectorial transport by NBCe1 might contribute to the transparency and hydration of lens similarly as in cornea.
Some studies identified the sodium bicarbonate cotransport activity that may originate from NBCe1 in the apical membrane of pigmented epithelium in frog, rat and frog retina [9,38-40]. NBCe1 in the apical membrane of pigmented epithelium may transport sodium and bicarbonate from sub-retinal space into the pigmented epithelium. The expression of NBCe1 in Müller cells was also detected, which might regulate local pH within retina [9,41].
NBCe1 was also found in human and rat pigmented and nonpigmented ciliary epithelium and in porcine nonpigmented ciliary epithelium [8,9,42]. In these epithelia, the functional evidence for anion exchanger has been suggested [43,44]. However, Shahidullah and colleagues reported that the production of aqueous humor was reduced by 4,4’-diisothiocyanatostilbene-2,2’-disulfonic acid and a carbonic anhydrase inhibitor acetazolamide in a low chloride buffer, suggesting that NBCe1 might be also involved in influx and efflux of bicarbonate into/from pigmented ciliary epithelium [42].
The presence of electrogenic sodium bicarbonate cotransporter activity in human trabecular meshwork cells was demonstrated by an electro-physiological experiment [45]. The immunohistochemical analysis also detected NBCe1 in human trabecular meshwork [8], suggesting that NBCe1 has some roles in trabecular meshwork cells.
The expression of NBCe1 was reported in other ocular tissues such as rat conjunctival epithelium and endothelium of human choriocapillaris [8,9,46]. However, the physiological significance of NBCe1 in these tissues remains unclear.
The immunohistochemical analysis and RT-PCR revealed the expression of NBCe1-A and NBCe1-B in human and rat cornea endothelium, human trabecular meshwork, rat lens epithelium, HLE cells, rat ciliary body and basal cells of rat conjunctiva [8,9,47]. However, the signals of NBCe1-A in RT-PCR were weaker than those of NBCe1-B, suggesting that NBCe1-B may be the main variant expressed in eye.
MUTATIONS OF NBCE1 AND OCULAR ABNORMALITIES
All the pRTA-related homozygous NBCe1 mutations except the NBCe1-A specific mutation Q29X cause band keratopathy. The inactivation of NBCe1 in corneal endothelium may reduce bicarbonate efflux from stroma into aqueous humor and increase bicarbonate concentrations in stroma. As shown in Fig. (3), the resultant increase in local pH may facilitate calcium deposition, resulting in band keratopathy. Sometimes EDTA chelation was tried for the treatment of this condition [24]. Histopathological observations of the cornea of band keratopathy associated with NBCe1 mutation have not been reported. A report analyzing band keratopathy associated with juvenile rheumatoid arthritis shows that precipitated calcium depositions are observed in Bowman’s layer or superficial lamellae of stroma [48].
Cataract was also found in all the homozygous mutations except Q29X. Although the importance of bicarbonate transport in lens epithelium remains to be established, NBCe1 might contribute to the transparency of lens.
Ten of twelve NBCe1 mutations developed glaucoma. Interestingly, Q29X also causes glaucoma, indicating that not only NBCe1-B but also NBCe1-A has an important physiological role in the pathogenesis of glaucoma. Although the heterozygous S982NfsX4 mutation developed normal tension glaucoma, the homozygous NBCe1 mutations usually developed open angle glaucoma with elevated intraocular pressure [22]. Glaucoma is the optic neuropathy characterized by death of a substantial number of retinal ganglion cells in the inner retina and loss of the optic nerve axons [49]. The definite mechanisms of glaucoma induced by NBCe1 mutations remain speculative. However, glaucoma is often associated with elevated intraocular pressure, and trabecular meshwork determines resistance of aqueous humor outflow [50]. Indeed, the contraction of trabecular meshwork cells is known to increase the outflow resistance and elevate intraocular pressure [51]. On the other hand, the contraction of trabecular meshwork cells is influenced by voltage-dependent L-type Ca2+ channels [52]. Therefore, the inactivation of electrogenic transporter NBCe1 may affect the contraction of trabecular meshwork cells by modifying the membrane potentials and intracellular Ca2+ concentration [8].
On the other hand, dysfunctional local pH regulation in the retina may be involved especially in the occurrence of normal-tension glaucoma by the heterozygous S982NfsX4 mutation. Indeed, retinal excitation by light is known to induce an increase in extracellular pH resulting in a gain of synaptic activity [53,54], and NBCe1 in retinal Müller cells may counteract this light-induced extracellular alkalosis [9,41]. Consistent with this speculation, the glutamate excitotoxicity was reported to facilitate the degeneration of retinal ganglion cells [55]. Interestingly, the association between normal-tension glaucoma and migraine has been reported [56]. Future studies will be required whether heterozygous NBCe1 mutations without pRTA may represent a risk factor for migraine and glaucoma.
Kao and colleagues recently reported that electrogenic sodium bicarbonate cotransporter 2, NBCe2 knockout in mouse caused loss of photoreceptors, ganglion cells and retinal detachment [57]. NBCe2 is expressed in whole body such as brain, heart, kidney, and muscle and considered to play important role in producing cerebrospinal fluid in choroid plexus [57-59]. Although functions of NBCe2 in retina remain unclear, it might also play an important role in maintaining retinal homeostasis.
ACKNOWLEDGEMENTS
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CONFLICT OF INTEREST
Declared none.


