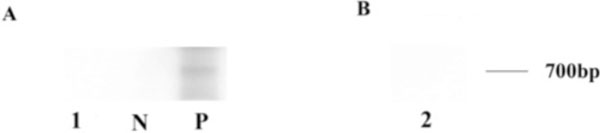RESEARCH ARTICLE
Classical Pathology of Sympathetic Ophthalmia Presented in a Unique Case
Shida Chen 1, Mary E Aronow 2, Charles Wang 3, Defen Shen 1, Chi-Chao Chan*, 1
Article Information
Identifiers and Pagination:
Year: 2014Volume: 8
First Page: 32
Last Page: 38
Publisher ID: TOOPHTJ-8-32
DOI: 10.2174/1874364101408010032
Article History:
Received Date: 20/3/2014Revision Received Date: 11/6/2014
Acceptance Date: 11/6/2014
Electronic publication date: 27/6/2014
Collection year: 2014

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
The ocular pathology of sympathetic ophthalmia is demonstrated in a 10 year-old boy who sustained a penetrating left globe injury and subsequently developed sympathetic ophthalmia in the right eye two months later. Two and a half weeks following extensive surgical repair of the left ruptured globe, he developed endophthalmitis and was treated with oral and topical fortified antibiotics. One month after the initial injury, a progressive corneal ulcer of the left eye led to perforation and the need for emergent corneal transplantation. The surgical specimen revealed fungus, Scedosporium dehoogii. The boy received systemic and topical anti-fungal therapy. Two months following the penetrating globe injury of the left eye, a granulomatous uveitis developed in the right eye. Sympathetic ophthalmia was suspected and the patient began treatment with topical and oral corticosteroids. Given the concern of vision loss secondary to sympathetic ophthalmia in the right eye, as well as poor vision and hypotony in the injured eye, the left eye was enucleated. Microscopically, granulomatous inflammation with giant cells was noted within a cyclitic membrane which filled the anterior and posterior chamber of the left globe. Other classic features including Dalen-Fuchs nodules were identified. Small, choroidal, ill-defined granulomas and relative sparing of the choriocapillaris were present. Molecular analysis did not identify evidence of remaining fungal infection. The pathology findings were consistent with previously described features of sympathetic ophthalmia. The present case is unique in that co-existing fungal infection may have potentiated the risk for developing sympathetic ophthalmia in the fellow eye.
1. INTRODUCTION
Sympathetic ophthalmia, a disease known since the time of Hippocrates, is a rare, bilateral, granulomatous uveitis that follows penetrating ocular trauma or surgical insult to one eye and threatens sight in the fellow eye which develops inflammation. The injured eye is referred to as the exciting eye, while the fellow eye is called the sympathizing eye. Ocular surgical procedures that disrupt the integrity of the uveo-retinal tissues have lately been shown to be among the important risk factors for developing sympathetic ophthalmia, however penetrating ocular trauma also plays a central role in the incidence of the disease [1]. It has been suggested that co-existing infection may increase the immune response due to greater exposure of retinal antigens and ultimately trigger an autoimmune process that leads to sympathetic ophthalmia [2-4]. While there seems to be no overall gender or racial predilection, the higher incidence of ocular trauma in males and children may explain the higher incidence of sympathetic ophthalmia in these groups reported in some series [5, 6]. Due to the rare nature of this disease, our current understanding of the pathophysiologic mechanisms underlying sympathetic ophthalmia is limited. Herein, we describe the classic ocular histopathology of a patient with sympathetic ophthalmia secondary to penetrating globe injury.
2. CASE REPORT
This study was approved by the National Eye Institute Institutional Review Board (IRB) for human subjects, and informed consent was obtained from the patient. A 10 year-old Mennonite boy sustained a traumatic perforation of the left globe due to a knife injury. He underwent extensive surgical repair including anterior chamber washout of 80% hyphema, lensectomy, vitrectomy for vitreous hemorrhage, and endolaser treatment for a posterior exiting wound in the infratemporal macula. Afterwards, he received intravitreal antibiotics including amikacin and vancomycin. Two weeks following the initial injury, he developed endophthalmitis. He underwent a vitreous and anterior chamber tap and was treated with intravitreal ceftazidime and vancomycin. He was also started on topical antibiotics including hourly besifloxacin and moxifloxacin. Cultures revealed rare fungal elements, but no bacteria. He was therefore started on topical hourly voriconazole and oral voriconazole 100 mg twice daily for 4 weeks. Due to concern for possible co-existing bacterial infection, oral doxycycline was added to his regimen. He developed a progressive corneal ulcer, and given the concern for Pseudomonas, standard topical antibiotics were switched to fortified topical hourly ceftazidime and vancomycin. One month after the injury, the corneal ulcer perforated, resulting in the need for emergent corneal transplantation in the left eye. The surgical specimen from the host cornea demonstrated fungal elements. DNA sequencing later revealed Scedosporium dehoogii. Seven weeks after injury, oral voriconazole was discontinued as there were no signs of residual infection. Two months following the penetrating globe injury of the left eye, granulomatous uveitis developed in the fellow, right eye. Sympathetic ophthalmia was suspected and the patient began treatment with topical prednisolone acetate 1% every 2 hours and oral prednisone (1.5 mg/kg/day) which was gradually tapered over the next 3.5 months. The vision in the left eye was light perception only. In the right eye, the keratic precipitates (KPs) on the endothelium and anterior lens capsule and cellular reaction in the anterior chamber did not respond to corticosteroid treatment. The vision in the right eye was 20/20. Ten weeks after the injury, enucleation of the left eye was performed given the concern for vision loss secondary to sympathetic ophthalmia in the right eye, as well as blindness and hypotony in the left eye.
Routine histopathology and immunohistochemistry were performed on the enucleated left globe. Immunohisto-chemistry was carried out using the avidin-biotin-complex immunoperoxidase technique with the Betazoid DAB Chromogen Kit (Biocare medical, CA, USA). Primary antibodies included CD68 (macrophage and microglia), CD3 (T-lymphocyte), and CD20 (B-lymphocyte) (Dako North America, Inc., Carpinteria, CA, USA). Secondary antibody was biotinylated goat anti-mouse IgG (1:200; Vector Laboratories, CA, USA). Microdissection was performed manually on uncovered stained glass slides, which were from formalin-fixed, paraffin-embedded tissue cut at 4-6µm. Tissue from an area of observed granulomatous inflam-mation in the region of the pupillary membrane and cyclitic membrane was microdissected for evaluation of fungal DNA. The microdissected tissue was immediately digested with Proteinase K buffer. Polymerase chain reaction (PCR) followed by gel electrophoresis was used to detect the amplification of fungal DNA. The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (IA, USA). Primers for 18S universal rDNA (Forward: 5-ATT GGA GGG CAA GTC TG-3; Reverse: 5-CCG ATC CCT AGT CGG CAT AG-3) and primers for Scedosporium dehoogii (MSDE1: 5- CGC CCG AAA GGA CGA CGG C-3; MSA2: 5-CTA CTC GAC TCG TCG AAG GAG C-3) were used. PCR amplification with 32P labeled primers was performed in a Peltier thermal cycler PTC 200 (M J research, Inc, Watertown, MA, USA) in a 10 µl-volume Eppendorf tube, containing 2 µl of extracted sample DNA, 1 µl of 10x Taq buffer, 1 unit of Taq DNA polymerase, 1 µl of MgCl2 (25 Mm), 2 µl of a four-deoxynucleoside triphosphate mix (10 mM each), and 2 µl of each primer (forward and reverse). The PCR amplification protocol was based on a previous publication with some modifications [7, 8]. For 18S universal rDNA primer: an initial five-minute denaturation at 95°C, followed by 45 cycles of 1 min denaturation at 95°C, 1 min annealing at 54.5°C, and 1 min extension at 72°C, final extension step was 5 min at 72°C. For Scedosporium dehoogii primers: 5 min denaturation at 95°C, followed by 35 cycles of 45 s denaturation at 94°C, 45 s annealing at 65°C, and 1 min extension at 72°C, final extension step was 10 min at 72°C. The amplification products were analyzed by 15% polyacrylamide gel electrophoresis in TBE buffer. Following gel electrophoresis and overnight autoradiography, the PCR products were visualized using a molecular imager (Bio-Rad Laboratories, CA, USA).
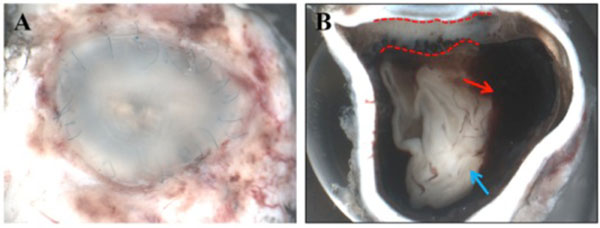
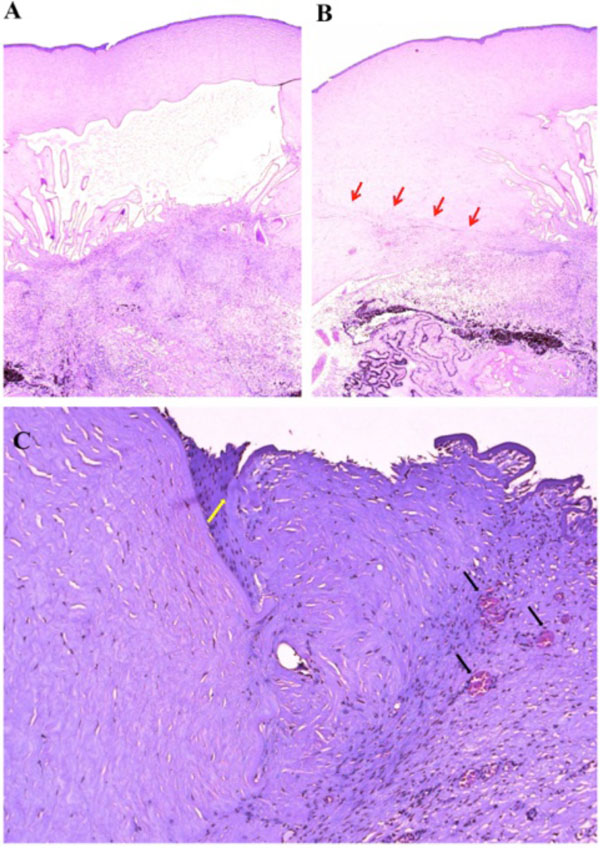
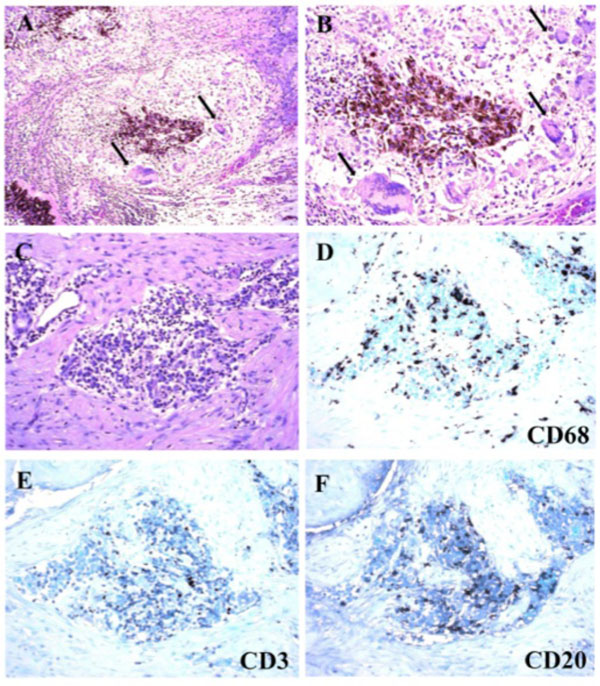
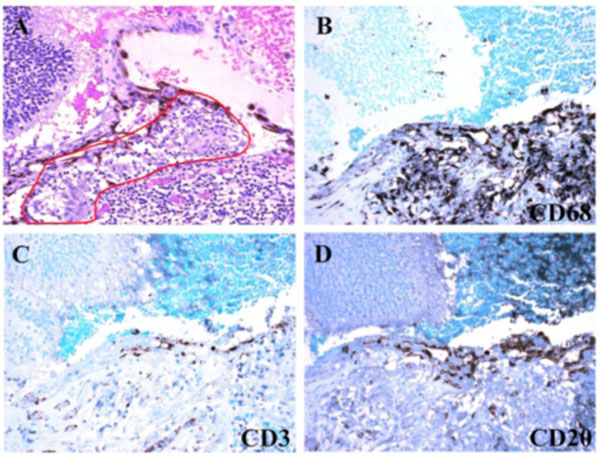
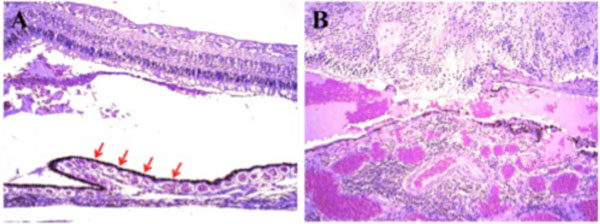
Macroscopically, a large corneal transplant button with corneal sutures was noted (Fig. 1A). There was a thick, proliferative, fibrous membrane in the pupillary area. The retina was totally detached and formed a funnel configuration, admixed with subretinal hemorrhage (Fig. 1B). Microscopically, corneal scar tissue was noted circumferentially along the peripheral corneal margin (Fig. 2A, B). There were Bowman’s membrane fragments and thick, dense, fibrous tissue with inflammatory cells and small neovascular lumens present within the corneal scar (Fig. 2C). The anterior chamber was shallow and contained erythrocytes and lymphocytes. The anterior chamber angle was narrow with peripheral anterior synechiae present as well as a dense pupillary membrane (Fig. 2A). Posterior synechiae from the necrotic iris were also adherent to the cyclitic membrane. Granulomatous inflammation containing many CD68+ macrophages and some multi-nucleated giant cells surrounded by lymphocytes (CD3+ > CD20+ cells) was present within the pupillary membrane, the cyclitic membrane, and the anterior vitreous (Fig. 3). There were a few lens capsule fragments and lens cortex remnants within the peripheral cyclitic membrane (Fig. 4A, B). The retina was totally detached in a funnel-shaped configuration, and appeared relatively intact in many areas. Dalen-Fuchs nod-ules were noted beneath the RPE layer (Fig. 5A, B) and they were composed of predominantly macrophages/epithelioid cells. Small, ill-defined granulomas were also seen in the choroid, along with some CD68+ macrophages, a few CD3+
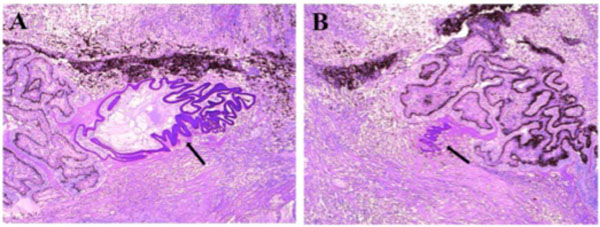 |
Fig. (4). Photomicrograph of lens capsule fragments and lens cortex. The lens remnants (black arrow) were located within the peripheral cyclitic membrane. (hematoxylin & eosin, x50). |
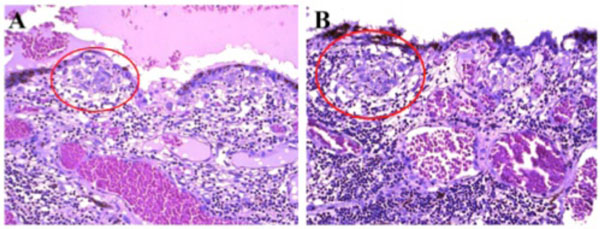 |
Fig. (5). Photomicrograph of Dalen-Fuchs nodules. Dalen-Fuchs nodules (red circle) were composed of mainly macrophages/epithelioid cells. (hematoxylin & eosin, x200). |
T lymphocytes, and rare CD20+ B lymphocytes (Fig. 6). The choroid had focal and diffuse monocytic infiltration, and the choroidal vessels were congested in some areas with overall relative sparing of the choriocapillaris (Fig. 7). No fungi were identified with Grocott-Gomori's methenamine silver stain (GMS). The tissues that demonstrated granulomatous inflammation were microdissected for PCR. However, no fungal DNA was detected using an 18S primer set or a primer set specific for Scedosporium dehoogii (Fig. 8).
3. DISCUSSION
The present case describes a 10 year-old child who sustained a penetrating knife injury to his left eye and two weeks later developed fungal endophthalmitis. Approximately two months following the injury, the fellow, right eye developed a granulomatous uveitis, and sympathetic ophthalmia was suspected clinically. The patient subsequently underwent enucleation of the injured left eye. The histopathology confirmed the diagnosis of sympathetic ophthalmia.
The histopathology of the patient’s eye demonstrated classic features of sympathetic ophthalmia. Widespread uveal granulomatous inflammation was present, which is a hallmark feature and is characterized by focal collection of macrophages and multi-nucleated giant cells surrounded by lymphocytes. Phacoanaphylaxis may also cause granulomatous inflammation and mimic sympathetic ophthalmia, however, phacoanaphylactic uveitis mainly involves the anterior segment with zonal granulomatous inflammation centered around the lens [3, 9]. In the present case, remnants of the lens capsule and cortex were observed, whereas they were not surrounded by intense inflammatory infiltration and did not induce phacoanaphylactic endophthalmitis. Dalen-Fuchs nodules are a classic feature of sympathetic ophthalmia, and are reported in 25%-35% of cases [10]. The Dalen-Fuchs nodules are predominantly located beneath the RPE layer, and are mainly composed of macrophages [11]. In some cases, when the disease is more advanced, these nodules may also contain depigmented RPE cells or lymphocytes [12, 13]. Previously described cases often report sparing of the retina and choriocapillaries, although one study demonstrated focal involvement of the choriocapillaris in 40% of cases [14]. In this same series, retinal detachment was observed in 50% of enucleated eyes, and this was associated with severe uveal inflammation [14].
Although, the exact etiology of sympathetic ophthalmia is not known, it has been hypothesized that altered tolerance to uveal and/or retinal antigens may be involved in the pathogenesis of this disease. One convincing theory suggests that the cell-mediated immune response to antigens from the retinal photoreceptor layer plays an integral role [15]. Implicated retinal antigens include: retinal soluble antigen (S-antigen) [16], rhodopsin [17], and interphotoreceptor retinoid-binding protein [18]. These antigens may induce a cell-mediated immune response. Lymphatics have also been suggested to play a role in the development of sympathetic ophthalmia [19, 20]. When ocular injury occurs, uveal tissue is exposed to conjunctival lymphatics, and antigens move to the regional lymph nodes, causing a cell-mediated immune response. In a rabbit model, subconjunctival injection of retinal S-antigen in one eye induced a bilateral sympathetic uveitis, however intraocular injection was ineffective in inducing sympathetic disease [20].
In early studies, it was suggested that a purulent eye infection such that occurs with penetrating ocular injury would destroy the uveal tissue and antigens to such an extent that this would prevent the development of sympathetic ophthalmia [10]. However, there are still a few case reports on the occurrence of sympathetic ophthalmia after endophthalmitis [2, 21]. Buller and associates reported a case of sympathetic ophthalmia following severe fungal keratitis, and hypothesize that chronic fungal infection allowed diffusion of intraocular microorganism antigens and proinflammatory mediators through a disturbed blood-retinal barrier to expose retinal antigens [2]. In one retrospective case series, 4 of 26 patients clinically diagnosed with sympathetic ophthalmia between 2002 and 2004, had concurrent bacterial endophthalmitis, which suggested that purulent endophthalmitis may not prevent the development of sympathetic ophthalmia [3]. The incidence of co-exiting endophthalmitis and sympathetic ophthalmia varies from 1% to 11% according to different studies [3, 10, 22]. One theory of pathogenesis suggests that co-exiting infection may increase the risk of development of sympathetic ophthalmia, because ocular infection provokes an immune response involving exposed retinal antigens and incites an autoimmune reaction [2, 3]. Experimental animal models have shown some evidence that bacterial products may function as an adjuvant entity which can enhance the uveitogenic potential of uveal and retinal antigens [23]. In our case, the GMS staining and fungal DNA detection studies using PCR in the enucleated eye were negative. This may be due to the fact that after one month of aggressive topical and oral anti-fungal treatment, and there were no signs of residual infection clinically.
Until now, there have been two major modalities employed in the clinical management of sympathetic ophthalmia: surgery (which includes either enucleation or evisceration aimed at prevention) and corticosteroid or immunosuppressive agents for treatment once disease develops. In this case, the injured eye was enucleated as there was concern for further vision loss of the fellow, right eye and no significant clinical improvement after the initiation of topical and oral corticosteroid treatment. There is controversy about the exact time to perform the enucleating surgery for the injured eye. Prompt action is generally recommended, because sympathetic ophthalmia has been reported as early as 5 days after injury and early enucleation of the injured eye has been shown to improve the visual prognosis of the sympathizing eye [10], Others question the appropriateness of preventative enucleation based on the fact that the exciting eye may actually retain better vision than the sympathizing eye in the course of the disease. Histologic studies have shown no benefit from enucleation of the exciting eye [24]. While significant controversy still exists regarding evisceration and enucleation, improved surgical techniques have resulted in a recent trend favoring evisceration due to improved cosmetic outcomes [15]. Regarding the importance of concurrent infection, early diagnosis of co-existing mixed infectious and inflammatory processes is important, and the prompt initiation of aggressive antimicrobial treatment as well as systemic corticosteroids may improve the prognosis in such cases [3].
CONCLUSION
This report demonstrated classic histopathologic features of sympathetic ophthalmia, which occurred secondary to a penetrating globe injury and concurrent fungal infection. This case may improve our understanding of the pathogenesis of sympathetic ophthalmia.
CONFLICT OF INTEREST
The authors declare no conflict of interest. Informed consent was provided from the patient.
ACKNOWLEDGEMENTS
The NEI Intramural Program provided funding support.