RESEARCH ARTICLE
The Causes of Hyperreflective Dots in Optical Coherence Tomography Excluding Diabetic Macular Edema and Retinal Venous Occlusion§§
Burak Turgut*, Hakan Yildirim
Article Information
Identifiers and Pagination:
Year: 2015Volume: 9
First Page: 36
Last Page: 40
Publisher ID: TOOPHTJ-9-36
DOI: 10.2174/1874364101509010036
Article History:
Received Date: 26/1/2015Revision Received Date: 18/2/2015
Acceptance Date: 5/3/2015
Electronic publication date: 31 /3/2015
Collection year: 2015

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Purpose :
To investigate the causes of hyperreflective dots (HRDs) in spectral domain optical coherence tomography (OCT) excluding diabetic macular edema (DME) and RVO (retinal vein occlusion).
Patients and Methods :
The medical records of 56 patients with HRDs documented by OCT were reviewed retrospectively. The patients with DME and RVO were excluded from the study in order to prevent misdiagnosing hard exudates or HRDs. The causes, unilaterality or bilaterality of HRD and demographic properties of the patients with HRD were evaluated.
Results :
Sixty four eyes of 56 patients having HRDs were included in this study. Of the patients with HRD, 17 (30.36%) were women and 39 (69.64%) were men. The ages of patients were between 13 to 84 years (median 60.18 years). The causes of HRD were as follows: papilledema in 4 eyes (6.25%), active neovascular age related macular degeneration (AMD) in 33 eyes (51.56%), familial dominant drusen in 2 eyes (3.13%), central serous chorioretinopathy in 19 eyes (29.69%) and commotio retina in 2 eyes (3.13%), choroidal folds in one eye (1.56%), branch retinal artery occlusion in one eye (1.56%), central retinal artery occlusion in one patient (1.56%) and Best vitelliform macular dystrophy in one eye (1.56%). The most common cause of HRD was AMD. The causes of HRDs in both eyes were AMD and papilledema.
Conclusion :
The most common causes of HRDs excluding DME and RVO seem as active exudative AMD. The presence of HRDs in retinal diseases might affect the decisions and the results of the treatment and the prognosis of diseases.
BACKGROUND
Spectral domain optical coherence tomography (SD-OCT) is a very useful non-invasive imaging method which is used in the diagnosis and follow-up of diseases involving the macula. It provides detailed information for the evaluation of drusen, intraretinal/subretinal hemorrhage or fluid and detachment of retina pigment epithelium (RPE) or retina [1-4]. Recently, Coscas et al. [5-7] found a new hyperreflective sign in SD-OCT previously unseen intraretinal changes and they called it as the hyperreflective dots (HRDs). They suggested that the presence of HRDs could affect the prognosis and treatment decisions, particularly in the patients with age related macular degeneration (AMD). Coscas et al. detected that HRDs scattered throughout all retinal layers, primarily around fluid accumulation in the intraretinal cystoid spaces. However, subsequently, HRDs have also been reported in retinal vein occlusion (RVO) and diabetic macular edema (DME) [7-10]. HRDs are not visible in any monochromatic and angiographic photos. Thus, we excluded the patients with DME and RVO in order to prevent misdiagnosing hard exudates or HRDs [11, 12]. In our study,
we aimed to investigate the causes of HRDs in optical coherence tomography excluding DME and RVO.
PATIENTS AND METHODS
In the study, we retrospectively reviewed the medical records of 632 patients with exlusion criteria who underwent OCT examination for various macular pathologies at our university hospital. The files were retrieved in the databases of the OCT machines. Sixty four eyes of 56 patients having HRDs were included in this study. The patients underwent complete ophthalmic examination, fundus photography, fundus autofloresence (FAF) and angiography, and OCT. OCT examinations were performed using SD-OCT and scanning laser ophthalmoscope (SLO) (OCT/SLO; OTI/OPKO Inc, Toronto, Canada). The SD-OCT device used in our study had transverse and axial resolutions of 20 and 5–6 µm, respectively. The acquisition speed was set at 28000 A-scan/sec. Every B-scan was composed of 512 A-scans. In vivo OCT images with high-resolution were obtained and analyzed for the presence of singular round or oval HRDs. Additionally, the characteristics of the HRD were also evaluated in all SD-OCT scans obtained. The presence of HRD in OCT was defined as the presence of small focal hyperreflective lesions scattered mainly in outer retinal layers, but also spreading to all retinal layers, observed in at least one OCT B-scan with high signal/noise ratio [5, 6]. HRDs were detected using only OCT while they were not visible in any red-free or chromatic photos, FAF images and angiography images. HRDs were classified on the basis of localization (outer, middle, inner retinal layers or disseminated in all retinal layers).
This study was designed as an institutional, retrospective trial and followed the tenets of the Declaration of Helsinki.
No approval from the institutional ethic committee board was needed due to the retrospective nature of the study. Informed consents were obtained from each patient after explanation of the nature and possible consequences of the study.
Exclusion Criteria
The patients with DME, RVO, epiretinal membrane or vitreomacular traction documented by OCT, and media opacities such as corneal opacity, lens opacity, vitreous and preretinal hemorrhage, proliferative diabetic retinopathy, arterial macroaneurysm, x-ray irradiation, carotid artery obstruction, sickle cell hemoglobinopathies, and patients with history of previous intraocular surgery, macular laser photocoagulation, and intravitreal injection were excluded from the study.
The patients had underwent complete ophthalmic examination, including best-corrected VA measurement using the Early Treatment Diabetic Retinopathy Study chart, slit-lamp biomicroscopy with a +90 diopter noncontact lens, red-free and color fundus photography, fundus autofloresence, fundus florescein angiography, and OCT.
RESULTS
Sixty four eyes of 56 patients having HRDs were included in this study. Of the patients with HRD, 17 (30.36%) were women and 39 (69.64%) were men. The ages of patients were between 13 to 84 years (median 60.18 years). The causes of HRD were as follows: papilledema in 4 eyes (6.25%), active neovascular AMD in 33 eyes (51.56%), familial dominant drusen (FDD) in 2 eyes (3.13%), CSCR in 19 eyes (29.69%) and commotio retina in 2 eyes (3.13%), choroidal fold in one eye (1.56%), branch retinal artery occlusion (BRAO) in one eye (1.56%), central retinal artery occlusion (CRAO) in one patient (1.56%) and Best’s vitelliform macular dystrophy (BVMD) in one eye (1.56%) (Table 1). The most common causes of HRD were AMD and CSCR. The causes of HRDs in both eyes were AMD and papilledema. All neovascular AMD cases, HRD were at active phase and were located outer retinal layers. In the present study, all CSCR patients have seen HRD were an acute phase of the disease with serous macular detachment (SMD). In these cases, HRDs were located at outer retinal layers just over SMD. In the cases of commotio retina, HRDs were detected at middle retinal layers. HRDs were dominantly located outer retinal layers in the eyes with papilledema, BRAO, CRAO, FDD, choroidal folds and BVMD. In the case with BVMD, HRDs were arranged in outer retinal layers over SMD as seen at CSCR. The diameters of HRDs were ranging between 10 and 70 micrometers on SD-OCT images. HRDs having small diameters were usually located in the inner retinal layers while large HRDs were located in the outer retinal layers and they created the high shadowing effects. The samples of OCT scan from the eyes with HRD were given in Figs. (1-5).
 |
Fig. (1). Sample optical coherence tomography scans from patients with age related macular degeneration. The arrows indicate hyperreflective dots. |
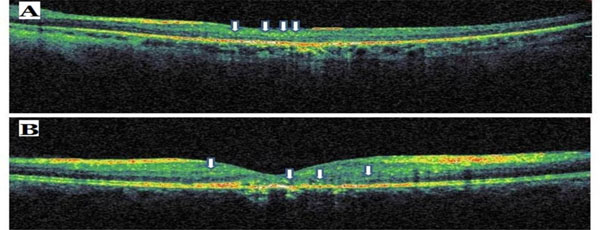 |
Fig. (2). Sample optical coherence tomography scans from patients with commotio retina (A) and central retinal artery occlusion (B). The arrows indicate hyperreflective dots. |
 |
Fig. (3). Sample optical coherence tomography scans from patients with familial dominant druzen (A), papilledema (B) and branch retinal artery occlusion (C). The arrows indicate hyperreflective dots. |
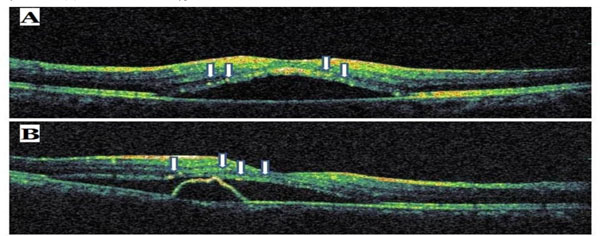 |
Fig. (4). Sample optical coherence tomography scans from patients with central serous chorioretinopathy. The arrows indicate hyperreflective dots. |
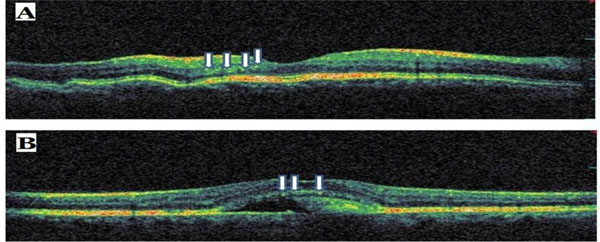 |
Fig. (5). Sample optical coherence tomography scans from patients with choroidal folds (A) and Best vitelliform dystrophy (B). The arrows indicate hyperreflective dots. |
The causes of hyperreflective dots in spectral domain optical coherence tomography.
| HRD Cause | Eyes (%) |
|---|---|
| Papilledema | 4 eyes (%6.25) |
| Active neovascular AMD | 33 eyes (51.56%) |
| FDD | 2 eyes (3.13%) |
| CSC | 19 eyes (29.69%) |
| Commotio retina | 2 eyes (3.13%) |
| Choroidal folds in | 1 eye (1.56%) |
| BRAO | 1 eye (1.56%) |
| CRAO | 1 eye (1.56%) |
| BVMD | 1 eye (1.56%) |
HRDs, hyperreflectice dots; AMD, age related macular degeneration; FDD, familial dominant drusen; CSC, central serous chorioretinopathy; CRAO, central retinal artery occlusion; BRAO, branch retinal artery occlusion; BVMD, Best vitelliform macular dystrophy.
DISCUSSION
The hyperreflective foci or dots are two different terms used to call abnormal hyperreflective elements detected in OCT scans. The HRDs are scattered, punctiform, small in size, mainly located in the outer retinal layers and/or around pockets of fluid accumulation and are typically not confluent [3].
HRDs are a new OCT findings demonstrated firstly by Coscas et al. [7]. It has been reported that they might seem in different retinal diseases, including AMD, DME, RVO, central serous chorioretinopathy, uveitis and macular telangiectasia [13-16]. They hypothesized that HRD were detected in the majority of patients affected by exudative AMD could be inflammatory activated and swelled cells or activated microglia cells because they may spread in all retinal layers in contrast to hard exudates, that they rapidly disappear after antiVEGF or anti-inflammatory treatment [7].
Although there are several theories of pathogenesis of HRD, its etiology is not clear. Some authors hypothesize that HRDs are focal accumulations of pigment or lipofuscin granules, while as others believe that they could be small intraretinal proteins or lipid exudates/deposits due to the breakdown of the blood-retinal barrier (BRB) in retinal vascular disease [7, 9, 16, 17]. Some different imaging modalities such as blue-light fundus photography and fundus autofluorescence eliminated that HRDs were not the focal accumulation of pigment or lipofuscin granules [7]. To another theory, it is considered that HRDs might be derived the degenerated photoreceptors or the macrophages fagosited them [16-19]. In a recent study, Uji et al. [19] demonstrated the presence of HRDs in the outer retinal layers and especially in the inner retinal layers in eyes with DME. They reported that HRDs in the outer retinal layers were closely associated with disrupted external limiting membrane and IS/OS line and decreased visual acuity.
Bolz et al. [9] reported that HRDs were distributed throughout all retinal layers in DME. They demonstrated that HRDs which located at the border of the outer nuclear layer and within the outer plexiform layer in some cases are confluent. They hypothesized that HRDs might represent subclinical features of lipoprotein extravasation that act as precursors of hard exudates, as they were not observed on clinical examination, fundus photography, or fluorescein angiography, due to their small size [9].
Ogino et al. [8] reported the presence and distribution of HRS in RVO and that HRDs were present in all retinal layers. They demonstrated that HRDs were attached to the external limiting membrane in most of the eyes with serous retinal detachment. The authors did not find any sign of hard exudates. They also suggested that the HRDs seen in affected areas might explain the leakage of blood constituents, whereas the HRS around the outer plexiform layer in the unaffected areas in RVO were associated with the absorption of water and solutes [8].
Framme et al. [20] reported the presence of HRDs in patients with both focal DME and diffuse DME. After anti-VEGF treatment, the HRDs were the first features to disappear or to reduce significantly. Therefore, it has been hypothesized that HRDs represent a clinical marker of inflammatory response [16, 19].
In our study, we found that the most common causes of HRD were AMD and CSCR. The all eyes having HRDs were the eyes with neovascular AMD at active phase and they were located in outer retinal layers. We support to Coscas et al. [7] concerning that the HRDs might be activated inflammatory or microglia cells because it is considered that inflammation plays in the role at the pathogenesis of AMD. In the present study, all CSCR patients with HRDs were an acute phase of the disease with serous macular detachment (SMD). HRDs were located at outer retinal layers just over SMD. We observed that HRDs were at middle retinal layers in the cases of commotio retina. HRDs were dominantly located outer retinal layers in the eyes with papilledema, BRAO, CRAO, FDD, choroidal folds and BVMD. In the case of the BVMD, HRDs were arranged in outer retinal layers over SMD as seen at CSCR.
It is known that CSCR and BVMD are non-inflammatory diseases. So, it is possible that HRDs seen in these diseases might be activated intraretinal microglia cells or macrophages phagosited outer segments of damaged photoreceptors. On the other hand, the cases with FDD, choroidal folds and commotio retina, HRDs might be due to inflammatory cells because of possible inflammatory pathogenesis of these disorders. In the cases of papilledema, BRAO and CRAO, the cause of HRDs might be the photoreceptor damage or the ischemia of the inner retinal layers.
In conclusion, the most common causes of HRDs excluding DME and RVO seem as active exudative AMD. The presence of HRDs in retinal diseases might affect the decisions and the results of the treatment and the prognosis of diseases.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Burak Turgut, MD, Department of Ophthalmology, Fırat University Hospital: conception, design, editing, analysis and review of the manuscript.
Hakan Yildirim, MD, Assistant of Ophthalmology, Fırat University Hospital: conception, design, typing, interpretation of data and review of the manuscript.






