RESEARCH ARTICLE
Spectral-Domain Optical Coherence Tomography for Glaucoma Diagnosis
Carolina P.B Gracitelli 1, 2, Ricardo Y Abe 1, 3, Felipe A Medeiros*, 1
Article Information
Identifiers and Pagination:
Year: 2015Volume: 9
First Page: 68
Last Page: 77
Publisher ID: TOOPHTJ-9-68
DOI: 10.2174/1874364101509010068
Article History:
Received Date: 28/3/2015Revision Received Date: 30/3/2015
Acceptance Date: 30/3/2015
Electronic publication date: 15/5/2015
Collection year: 2015

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Identification of structural damage to the optic nerve and retinal nerve fiber layer (RNFL) is an essential component of diagnosis and management of glaucoma. The introduction of spectral-domain OCT (SD-OCT) has allowed objective quantification of damage to these structures with unprecedented resolution. In addition, recent attention has been directed towards imaging the macular area for quantifying loss of neural tissue caused by the disease. Many studies have evaluated and compared the diagnostic accuracies of a variety of parameters that can be obtained from imaging these areas of the ocular fundus. In this article, we critically review the existing literature evaluating the diagnostic accuracy of SD-OCT in glaucoma and we discuss issues related to how SD-OCT results should be incorporated into clinical practice.
INTRODUCTION
Glaucoma is an optic neuropathy characterized by progressive loss of retinal ganglion cells (RGCs) and associated morphological changes to the optic nerve and retinal nerve fiber layer (RNFL) [1]. Loss of visual function in glaucoma is generally irreversible and, without adequate treatment, the disease can progress to disability and blindness [1]. Although visual field testing has been widely used for diagnosis, staging and monitoring the disease, in many patients visual field losses only become detectable after a substantial number of RGCs has been lost [2-11]. Previous studies have reported that from 25% to 35% of RGCs would need to be lost on average for statistically significant abnormalities to appear on standard automated perimetry (SAP) examinations [12, 13]. Therefore, early identification of structural damage to the optic disc and RNFL is paramount for an early diagnosis of the disease [9, 14-17].
The use of spectral domain optical coherence tomography (SD-OCT) technology has enabled clinicians to obtain high-resolution images of the optic nerve head (ONH), RNFL and macular regions [18]. Several studies have been published evaluating the accuracy of different structural measurements obtained with SD-OCT for diagnosing glaucoma [19-23]. In this review, we critically evaluate the existing literature on this topic. We also review issues related to how diagnostic tests should be evaluated in glaucoma and how SD-OCT results should be incorporated into clinical practice.
DIAGNOSTIC ACCURACY OF SD-OCT IN GLAUCOMA
Until a few years ago, clinically available OCT instruments used a technique referred to as time-domain OCT (TD-OCT) to obtain images of the ocular fundus. Although RNFL thickness measurements obtained with TD-OCT have been shown to discriminate normal eyes from those with glaucoma [8, 24-29] and to detect change over time [8, 30, 31], this technology was limited by a suboptimal resolution and slow scan acquisition times. The introduction of SD-OCT improved resolution and scan acquisition time compared to TD-OCT, leading to better reproducibility and accuracy in quantifying structural damage in glaucoma [22, 32-34].
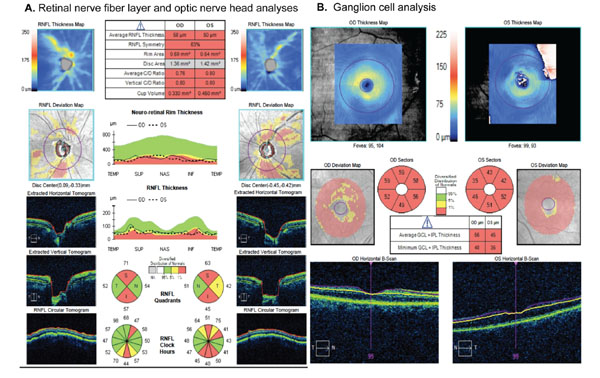
There is an extensive list of studies that have investigated the diagnostic accuracy of SD-OCT RNFL parameters in glaucoma [21, 23, 35-38]. RNFL thickness parameters evaluated in these studies have generally included the global average peripapillary RNFL thickness, corresponding to the average of all thickness measurements in the peripapillary circle around the ONH, as well as parameters measuring thickness by quadrants (superior, inferior, temporal, nasal) or in small clock-hour sectors [21, 23, 35-38]. Fig. (1) shows a printout of RNFL analysis provided by a commercially available SD-OCT instrument (Spectralis SD-OCT, Heidelberg Engineering, Dossenheim, Germany) for a glaucomatous patient with diffuse RNFL loss in the right eye and normal RNFL in the left eye.
Depending on the specific parameter evaluated and the characteristics of the studied population, sensitivities for detection of glaucomatous damage by the best performing RNFL parameters have been reported to range from approximately 60% to 98%, for specificities ranging from 80% to 95% [21, 23, 35-38]. Areas under the receiver operating characteristic (ROC) curves, a summary index of diagnostic accuracy, have been reported to range from 0.81 to 0.98 [21, 23, 35-38]. In general, the parameters with best diagnostic accuracy have been the average peripapillary RNFL thickness and thicknesses in the inferior and superior quadrants [21, 23, 35-38]. This is in agreement with previous studies demonstrating that the superior and inferior areas of the optic nerve are most commonly affected in glaucoma [39-41]. It should be noted that, while sectorial RNFL parameters may increase the chance of detecting localized RNFL damage in glaucoma, these parameters frequently suffer from low reproducibility, as measurements are averaged over only relatively small areas [21, 23, 35-38]. On the other hand, the global average RNFL thickness has generally been shown to be the most reproducible parameter, which is not surprising considering that its calculation involves averaging measurements over a relatively large area. The improved reproducibility offers large gains in the ability to detect progression over time. The gain in reproducibility, however, may come at the expense of potentially missing few localized RNFL defects, especially in cross-sectional evaluations. However, even for detection of localized RNFL defects, it seems that global RNFL thickness measurements still perform at least as well as sectorial parameters, suggesting that the improved reproducibility may overcome the limitation of averaging measurements over a large area [42, 43].
In recent years, increased attention has been directed toward the macular region for evaluation of glaucomatous damage. As a large proportion of total macular thickness is composed of RNFL and ganglion cell bodies, this region is an attractive area for identifying structural damage from the disease [44]. The macular RGC layer contains more than 50% of the RGCs of the entire retina [45]. Furthermore, at least in eyes without macular pathologies, there appears to be less variability and a lower likelihood of anomalous structural characteristics compared with the optic disc and peripapillary region [44, 46]. Recent studies have also suggested that, in contrary to previous belief, glaucomatous damage frequently affects the macular region leading to central visual field losses that can go undetected [47]. A recent investigation also demonstrated that glaucomatous RGC damage to the macular area seems to occur at the same proportion to the damage seen in regions outside the macula [48].
SD-OCT allows quantitative assessment of either the entire macular thickness or of thickness of specific layers that may be important in glaucoma [49, 50]. Parameters available from SD-OCT analysis of the macular area include, for example, the macular RNFL, the ganglion cell layer with the inner plexiform layer (GCIPL) and the so-called ganglion cell complex (GCC), which has been described as comprising the RNFL, the ganglion cell layer, and the inner plexiform layer [49, 50]. Fig. (2) shows printouts with measurements obtained from different scanning areas (RNFL, ONH and macula) with one of the commercially available SD-OCT instruments, the Cirrus-OCT (Carl Zeiss Meditec, Dublin, CA, USA).
Several studies have shown that macular parameters are able to distinguish glaucomatous eyes from those of healthy subjects [51-53]. Kim et al. [51] found that RNFL and GCC thickness had a similar diagnostic performance in detecting early, moderate and advanced glaucoma. In another study, Cho et al. [54] reported similar correlations between visual field mean sensitivity, GCC, and RNFL thickness in glaucomatous eyes. In their report, they found that for early visual field defects, the GCC showed a discrimination capacity comparable to that of RNFL parameters.
The SD-OCT is also able to provide topographical measurements of the ONH, including optic disc area, neuroretinal rim area and volume, as well as cup area and volume. Although previous versions of the OCT technology were also able to provide such measurements, a large amount of data interpolation was required, resulting in poor reproducibility and accuracy of the measurements [55, 56]. The improved resolution and velocity of scan acquisition of SD-OCT has greatly reduced the need for interpolation, resulting in much better delineation of the ONH structures. The utility of SD-OCT ONH parameters for glaucoma diagnosis, however, has not been well established. Although some studies have shown these parameters are similar [57] others have reported that they are inferior to RNFL measurements for glaucoma detection [58]. It is likely that the differences in these results are consequence of the reference standard used to select the populations with glaucomatous damage and controls. As in any diagnostic accuracy study, some sort of reference standard needs to be employed to select cases and controls [59]. The ability to distinguish cases and controls, as defined by the reference standard, is then investigated for the parameters under evaluation, such as RNFL thickness, neuroretinal rim area or cup/disc ratio. Most studies have included as reference criteria for cases the requirement to have glaucomatous field losses with “compatible” optic nerve damage, while controls must have “normal appearing optic nerves”. If the reference criteria for diagnosis include an assessment of the optic disc by clinicians using slit-lamp fundoscopy or optic disc photographs, this increases the chances that those patients with clear abnormalities on optic disc features such as rim or cup will be those selected for inclusion as cases in the study, as opposed to those with RNFL abnormalities. This occurs because optic disc features such as cup size or rim thinning are more easily visible and detected as abnormal by clinicians than abnormalities seen in the RNFL [60, 61]. A similar effect is seen in the selection of controls. Controls are frequently selected because they have “normal optic discs”, which in essence ends up being a selection of individuals who have normal appearing neuroretinal rim and cup. Such selection criteria can strongly bias some studies toward favoring the accuracy of topographic optic disc-based parameters and is frequently a major source of misinterpretation and misuse of imaging in clinical practice [59].
Using visual field defects as reference standard, Rao et al. [35] evaluated the accuracies of the RNFL, ONH, and macular thickness scanning protocols obtained by SD-OCT to differentiate normal eyes from eyes with glaucomatous field defects and found that the RNFL and inner retinal macular thickness measurements had good diagnostic accuracy, with ROC curve areas of 0.88 and 0.87, respectively. The RNFL and macular parameters performed significantly better than the best ONH parameter, which had an ROC curve area of 0.81. It should be noted, however, that there are also limitations from using visual fields as reference standard in diagnostic accuracy studies in glaucoma, as discussed later in this article.
It should be noted that, although imaging technologies have a good ability to detect glaucoma, the diagnostic performance decreases for detection of early disease compared to moderate or advanced disease [62]. Leite et al. [63] reported that in patients with minimal visual field losses, average RNFL thickness had sensitivity of 48% for specificity at 95% (ROC curve area= 0.82), while the sensitivity increased to 84% at the same specificity (ROC curve area = 0.96) for patients with moderate visual field loss [63].
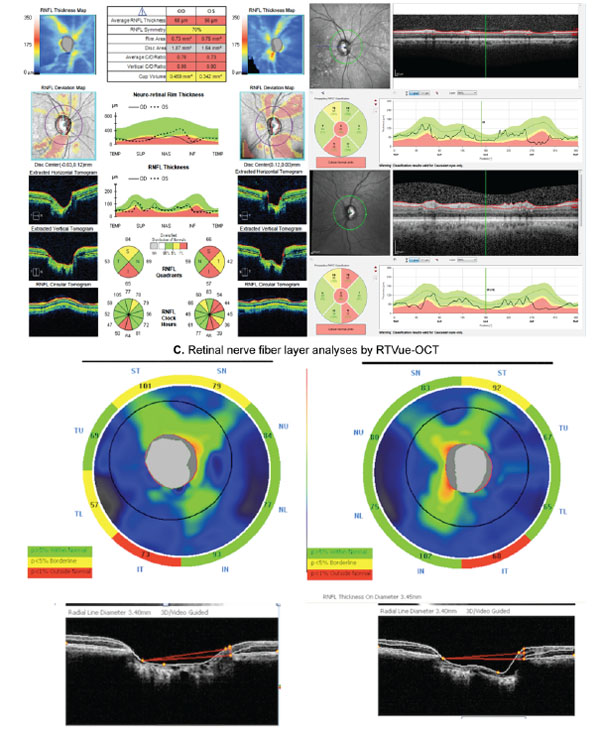
Several different SD-OCT instruments are commercially available. Although their measurements do not appear to be interchangeable [64], they have been demonstrated to have similar diagnostic capabilities in cross-sectional investigations [36, 65]. Leite et al. [36] analyzed and compared the diagnostic accuracies of RNFL thickness using Spectralis SD-OCT, Cirrus-OCT and RTVue-OCT (Optovue Inc., Fremont, CA, USA). Although SD-OCT instruments have different resolution and acquisition rates, Leite et al. showed that their ability to detect glaucoma in a cross-sectional investigation was very similar. Akashi et al. [65] found similar results when comparing diagnostic abilities of three different SD-OCTs (Cirrus-OCT, RTVue-OCT and 3D-OCT [Topcon Corporation, Tokyo, Japan]). Fig. (3) illustrates the RNFL thickness analyses from a glaucoma patient analyzed on the same visit date with three different SD-OCTs: Cirrus-OCT, Spectralis SD-OCT, and RTVue-OCT. The patient had an inferior RNFL defect, which was clearly detectable by all three instruments.
DETECTING GLAUCOMATOUS DAMAGE WITH SD-OCT IN EYES SUSPECTED OF HAVING THE DISEASE
Most of the studies evaluating the diagnostic accuracy of SD-OCT have evaluated the instrument’s ability to discriminate eyes of patients with repeatable glaucomatous visual field defects from those of healthy subjects [59, 66, 67]. Such studies are important for providing an initial exploratory evaluation of newly developed methods to detect glaucomatous damage [59, 66, 67]. For example, if a test fails to differentiate cases from controls at this stage, no more evaluations would be performed, and the test would be generally considered useless. If properly done, these studies may also overcome certain biases related to the selection criteria for cases and controls, as discussed above. However, in clinical practice, a diagnostic test is used to diagnose disease in patients suspected of having the disease, not in patients with a confirmed diagnosis. Therefore, from a practical standpoint, it seems of little utility to demonstrate that an imaging device is able to diagnose glaucoma in a patient with a confirmed visual field defect; because such patient would already have a clear diagnosis established. In fact, in a study evaluating the impact of design-related bias in studies of diagnostic tests in glaucoma, Medeiros et al. [68] found that studies with a case-control design that includes patients with well-established disease and a separate group of normal (unsuspected) control subjects substantially overestimate the performance of the tests. Therefore, if a test succeeds in initial exploratory diagnostic studies, further steps are necessary to evaluate whether it is able to provide clinically relevant information. That is, one needs to demonstrate that the new diagnostic test can be helpful in clarifying the diagnosis in those suspected of having damage, as opposed to those who can be clearly diagnosed based on currently existing standard tests. The optimal design for assessing a diagnostic test’s accuracy is considered to be a prospective, blind comparison of the test and the reference test in a consecutive series of patients from a relevant clinical population; that is, those suspected of having the disease [66].
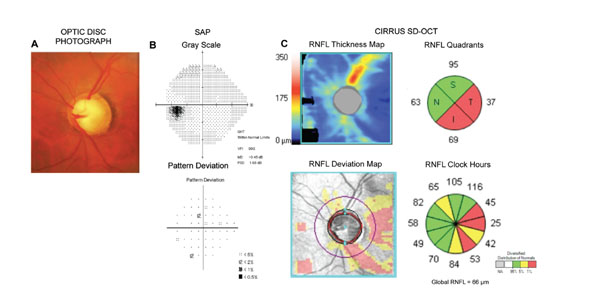
Lisboa et al. [69] evaluated the diagnostic accuracy of RNFL with Spectralis SD-OCT to detect damage in patients suspected of having glaucoma [69]. The study examined 134 eyes of 88 subjects who were suspected of having glaucoma because of their optic disc appearance, but who had normal visual fields at the time of OCT testing. This design, therefore, replicates the situation frequently faced by clinicians in attempting to diagnose early glaucomatous damage in subjects who present suspicious findings from the disease but in whom the visual fields are still not clearly abnormal. A challenge of such studies is that, as only suspect patients are included, one needs to find a way of establishing whether or not glaucomatous damage is actually present without relying on visual fields. An approach to overcome this difficulty was suggested by Medeiros et al. [70]. The authors used previous documented history of changes to the optic nerve in order to establish the reference diagnosis and divide patients in those with glaucoma versus controls. If a subject had clear evidence of progressive optic nerve damage on stereophotographs, this subject could be classified as having glaucoma, despite still presenting with normal visual fields (preperimetric glaucoma). Of note, the documented history of progressive damage had to be present before the imaging test date, in order to guarantee that these patients could be safely deemed as glaucomatous by the time they had their imaging test done. The control group was also composed of eyes suspected of having glaucoma, however, these eyes had been followed for a very long period of time (more than ten years) without treatment and without any evidence of development of optic nerve damage on photos or visual field. The long-term follow-up without any detectable change gives confidence that these control eyes were most likely normal, despite having suspicious optic nerve appearance. This design avoids biases introduced by selecting control eyes based on “normal appearing optic discs”, as discussed above. In addition, it allows for the assessment of a homogenous cohort of eyes suspected of having glaucoma, with an evaluation of diagnostic accuracy in a situation of direct clinical relevance. The investigators found that SD-OCT was able to discriminate eyes with preperimetric glaucoma from those with suspected glaucoma [69]. The best performance was obtained by the superior temporal, global and inferior temporal RNFL thicknesses [69]. The ROC curve areas for these parameters were 0.88, 0.86 and 0.81, respectively [69]. Fig. (4) shows an example of an eye with preperimetric glaucoma diagnosed using RNFL measurements obtained by SD-OCT.
In another study, Lisboa et al. [71] compared the diagnostic ability of RNFL, ONH and macular parameters to diagnose preperimetric glaucoma. Their study had a similar design as the one described above, including a cohort of suspect patients that had been followed for an average of 13 years. The investigators demonstrated that the RNFL parameters performed significantly better than did the ONH and macular parameters. Average RNFL thickness had better ability to detect preperimetric glaucoma compared with vertical C/D (cup-to-disc) ratio (ROC curve areas of 0.89 vs 0.74, respectively; P = 0.007) and GCC average thickness (0.89 vs 0.79; P = 0.015).
Recently, in a retrospective cross-sectional study, Kim et al. [72] evaluated the diagnostic accuracy of macular inner retinal layers and RNFL parameters of the Topcon 3D SD-OCT. They evaluated 64 healthy eyes, 68 eyes with preperimetric glaucoma, and 72 eyes with early glaucomatous visual field losses. Eyes with preperimetric glaucoma were defined as those with normal visual field results, but with one or more localized RNFL defects (on red-free fundus photographs) or with history of documented evidence of progression. The Topcon 3D-OCT’s ability to detect preperimetric glaucoma as measured by the ROC curve area was similar for RNFL thickness (0.77), GCIPL (0.73) and GCC parameters (0.72). The same authors used identical methodology to evaluate the performance of macular GCIPL thickness for detecting preperimetric glaucoma with the Cirrus-OCT and found that the diagnostic ability of the macular GCIPL parameters was comparable to that of peripapillary RNFL and ONH parameters [73].
COMBINING STRUCTURE AND FUNCTION FOR DIAGNOSIS OF GLAUCOMA
Although many patients with glaucoma show signs of structural damage before full development of statistically significant abnormalities on SAP [3-5, 7-11, 74, 75], others may show evidence of functional deterioration without measurable changes in structural tests [5, 11, 75]. This imperfect relationship between structural and functional measurements seems to be derived largely from the different algorithms, measurement scales and variability characteristics of the tests. In fact, Harwerth et al. [76] demonstrated that structural and functional tests are in good agreement as long as one uses appropriate measurement scales for neural and sensitivity losses and considers factors such as the effect of aging and eccentricity on estimates of neural losses [76]. In a series of investigations, they demonstrated that RGC loss estimates obtained from clinical perimetry agreed closely with the estimates of RGC losses obtained from RNFL assessment with OCT [76]. Realizing that estimates of RGC loss could provide a common domain for expressing the results of structural and functional tests, Medeiros et al. developed the idea of combining these estimates from different tests in order to develop a single index of structure and function that could improve the reliability and accuracy for estimating neural loss in glaucoma [16, 77]. The method combines RGC count estimates from OCT and SAP and averages them using a weighting system that considers differences in the performance of SAP and imaging tests at different stages of the disease [16, 77]. The weighted RGC estimate has been used to develop a combined structure-function index (CSFI) [78]. The CSFI is an estimate of the percentage of RGCs loss compared with the age-expected RGC number obtained by comparison to a normative database [78]. Thus, an eye with a CSFI of 100% has an estimated RGC count equal to that expected for age, whereas an eye with a CSFI of 50% has an estimated RGC count half that expected for age [78]. The purpose of the CSFI is to merge the results of structural and functional tests into a single index that can be used to diagnose, stage and detect disease progression.
The performance of the CSFI to diagnose glaucoma was evaluated in a cross-sectional study involving 333 glaucomatous eyes and 330 eyes of healthy subjects [78]. Among the glaucomatous eyes, 295 (89%) had perimetric glaucoma, and 38 (11%) had preperimetric glaucoma. The mean CSFI, representing the mean estimated percent loss of RGCs, was 41% in the perimetric group and 17% in the preperimetric group. The index had excellent diagnostic performance to detect glaucomatous eyes, with an area under the ROC curve of 0.94. The index was also able to successfully detect eyes with preperimetric glaucoma, with an area under the ROC curve of 0.85. The index performed better than isolated measures of structure and function for diagnosing preperimetric and perimetric glaucoma. In addition, by combining structural and functional tests into a single estimate of RGC loss, the index provides an intuitive parameter for clinical use.
It is important to note that the CSFI uses empirically derived formulas to estimate the number of RGCs from SAP and OCT based on previous experimental studies in monkeys [76]. However, although the estimates obtained from these formulas have been validated in multiple external cohorts, including human data [76], no studies have compared the actual CSFI estimates with histological estimates of human glaucomatous eyes. However, this concern may be of little practical relevance as long as CSFI measurements are demonstrated to be of clinical utility [78, 79]. In fact, very little histologic validation has ever been done for the variety of SD-OCT parameters that estimate the thicknesses of the different retinal layers.
DETECTING PROGRESSION WITH SD-OCT
The ability of any device to detect glaucomatous damage at any single point in time (cross-sectional evaluation) is limited. This occurs, at least in part, due to the considerable overlap that can exist between measurements from eyes with glaucoma and those from the normal reference population. There is a large range of measurements for the several parameters obtained by the SD-OCT in the normal population, resulting in relatively wide confidence limits of what can be considered normal. In many cases, a glaucomatous eye with significant loss of neural tissue over time may have measurements that are still considered within normal limits when evaluated at any single point in time. Therefore, the assessment of structural damage over time may be the only way to clearly establish the diagnosis of glaucoma in many eyes suspected of having the disease. Longitudinal evaluation of these eyes is essential in order to ensure that progressive damage can be detected, confirming the diagnosis even before the measurements fall outside normal confidence limits. Meira-Freitas et al. [79] used the CSFI to predict the development of glaucoma and visual field loss in 288 eyes of 288 subjects suspected of having glaucoma at baseline. Over a mean follow-up period of approximately 4 years, 48 eyes were deemed to have developed glaucoma based on repeatable abnormal visual fields or progressive glaucomatous optic disc changes on masked assessments of stereophotographs. Eyes with lower estimated number of RGCs at baseline and those with faster rates of change in RGC counts over time were found to be at greater risk of developing glaucoma (hazard ratio = 1.56 per 100,000 cells lower at baseline; P = 0.002 and hazard ratio = 2.68 per 10,000 cells/year faster rate of loss; P = 0.014, respectively) [79]. The results of this study showed the importance of longitudinally following glaucoma suspects over time in order to clarify the diagnosis and establish risk [79].
A review on the ability of SD-OCT in detecting glaucomatous progression is provided in a companion article [80].
LIMITATIONS OF SD-OCT
For proper use of SD-OCT in clinical practice, it is essential to be aware of the limitations of the technology and of currently available devices. Image results can be affected by artifacts, such as those produced by eye movements or by media opacities [81]. In addition, artifacts may be caused by failures in the SD-OCT algorithm that delineates the retinal layers [81]. Such errors may cause spurious measurements of the thicknesses of the different layers and structures of interest [81] [82]. Ocular diseases such as myopia [83], age-related macular degeneration [46], or the presence of macular drusen [46], may also introduce artifacts and confound the interpretation of results of certain parameters. Several studies have reported that topographic optic disc parameters and RNFL thickness measured with SD-OCT may be less effective in discriminating glaucomatous from nonglaucomatous subjects in eyes with high myopia [83].
CONCLUSION
The ability to detect and quantify structural damage is essential for proper diagnosis and management of glaucoma. Assessment of RNFL, macular and ONH damage with SD-OCT has been proven useful for diagnosing the disease at different levels of severity, as well as for quantifying risk in glaucoma suspects. In addition, approaches combining structural measurements from SD-OCT with functional assessment by perimetry may be advantageous compared to those using just single parameters from each test, offering a powerful way to detect neural losses in glaucoma and to evaluate disease deterioration.
CONFLICT OF INTEREST
FAM: Research support from Carl-Zeiss Meditec, Heidelberg Engineering and Topcon, Inc. Consultant to Carl-Zeiss Meditec, Inc. CPBG: None and RYA: None.
ACKNOWLEDGEMENTS
Supported in part by National Institutes of Health/National Eye Institute grants EY021818 (F.A.M.) and core grant P30EY022589; an unrestricted grant from Research to Prevent Blindness (RPB); Brazilian National Research Council-CAPES grant 12309-13-3 (CPBG).