RESEARCH ARTICLE
Dual Pathogenesis of Primary and Recurrent Pterygium: Immunohistochemical Proof
Doaa Ghorab1, Ahmed Helaly2, 3, *, Amani E. Badawi4
Article Information
Identifiers and Pagination:
Year: 2021Volume: 15
First Page: 229
Last Page: 235
Publisher ID: TOOPHTJ-15-229
DOI: 10.2174/1874364102115010229
Article History:
Received Date: 27/4/2021Revision Received Date: 15/7/2021
Acceptance Date: 27/7/2021
Electronic publication date: 19/11/2021
Collection year: 2021

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction:
Pterygium is a common ophthalmic problem in the Middle East where exposures to dust and sun rays are risk factors. The condition is more prevalent in middle-aged males and can be considered as an aging process. The aim of this study is to test both the degenerative and the proliferative components of Pterygium by both reduced glutathione and topoisomerase one activity.
Methods:
The study applied immunohistochemistry staining for both reduced glutathione and topoisomerase 1.
Results:
The samples expressed positive glutathione staining in most primary Pterygium conditions and all secondary Pterygium. On the other hand, the topoisomerase 1 immunohistochemistry expressed focal activity in secondary conditions suggesting a progenitor cell role in the pathogenesis of Pterygium in conjunction with oxidative stress.
Conclusion:
Pterygium represents dual pathology with a proliferative component and a degenerative one that needs further studies. It is possible to use combination immunohistochemistry markers to predict the prognosis of Pterygium behavior.
1. INTRODUCTION
Pterygium is a possible aging-related condition that is associated with collagen degeneration. This degeneration expressed fibro-vascular reaction creeping over the cornea. The pathogenesis of Pterygium is not clear. However, Sunrays and exposure to dust are risk factors for this condition. A widespread meta-analysis study on more than 400 000 candidates worldwide demonstrated increasing rates of Pterygium cases with age predominantly in males exposed to sun rays. The condition was aggravated in rural areas with the highest prevalence in China. Sunglasses and smoking were considered protective factors against Pterygium pathology [1, 2]. However, it is not explained how smoking improves the condition despite that sun rays and aging share the burden of oxidative stress with smoking.
Scientists proposed a multifactorial theory to explain Pterygium with possible genetic, immunological, and viral infection causes of the development of such pathology. On the other hand, it is important to notice that Pterygium has a degenerative component and a proliferation component at the same time [3]. The condition is benign in most cases, with minimal symptoms like foreign body sensation and burning.
Some studies considered Pterygium as a neoplastic process; they linked this tumor proliferation with p53 dysfunction and altered stem cell activity [4]. Progressive Pterygium growth passes to the cornea and impairs vision. At this point, surgical treatment is the standard gold method. Unfortunately, there is a recurrent rate of pathology that requires removal. Transposition of the conjunctival flap, conjunctival auto-transplantation, and amniotic membrane transplantation were common techniques to reduce the recurrence. Adjuvant drug therapy has been applied to reduce the risk of recurrence, like vascular endothelial growth factor inhibitors [5]. Conservative treatment is partially successful in non-fibrotic conditions [6].
Topoisomerase 1 is an important enzyme in DNA synthesis related to tumor progression [7]. Topoisomerase 1s are nuclear enzymes that release DNA rigidity allowing DNA replication. Topoisomerase 1 is a marker of cell proliferation and helps in the diagnosis of cancers. It is a target of anticancer therapy. This enzyme expression may explain the pathogenesis and the prognosis of proliferative disorders [8].
The current work will hopefully use immunohisto- chemistry to examine the two markers glutathione and topoisomerase 1 to explain the interaction between both degenerative and proliferative mechanisms occurring in the same disorder. Hopefully, it is suggested that immunohis- tochemistry can help in the prediction of the recurrence and possibly the mechanisms involved in this disorder.
2. PATIENTS AND METHODS
The current research work is a single-center retrospective study conducted at the Department of Ophthalmology, and the Department of Pathology, Mansoura University, Egypt, according to the tenets of the declaration of Helsinki. The article was accepted by the IRB of the faculty of Medicine, Mansoura University, code number R.20.01.728
The inclusion criteria were as follows: age 18 years or more, unilateral nasal primary or recurrent Pterygium, prominent growth, reported vascularization, uncontrollable irritation symptoms, and growth invasion of the corneal surface by about 2-4 mm. While, the exclusion criteria were: pseudo- Pterygium, ocular surface disorders (as severe dry eye), eyelids infection or infection of the ocular surface, patients reported active extensive intraocular inflammatory reaction, patients with ocular hypertension or on glaucoma under medical control, patients with advanced, recurrent Pterygium that extends between the pupil and limbus at the time of the presentation, and combined other eye surgical history within the previous last six months (Tables 1-3).
| Gender | Type of Pterygium | Total | |||
|---|---|---|---|---|---|
| Primary | Secondary | ||||
| Sex | Female | Count | 6 | 3 | 9 |
| % of Total | 22.2% | 11.1% | 33.3% | ||
| Male | Count | 14 | 4 | 18 | |
| % of Total | 51.9% | 14.8% | 66.7% | ||
| Total | Count | 20 | 7 | 27 | |
| % of Total | 74.1% | 25.9% | 100.0% | ||
| Primary | G0 | G1 | G2 | G3 |
|---|---|---|---|---|
| Male (13) | 3 | 4 | 2 | 4 |
| Female (7) | 1 | 2 | 2 | 2 |
| Secondary | ||||
| Male (4) | 0 | 0 | 2 | 2 |
| Female (3) | 0 | 0 | 0 | 3 |
| Primary | G0 | G1 | G2 | G3 |
|---|---|---|---|---|
| Male (13) | 13 | 0 | 0 | 0 |
| Female (7) | 7 | 0 | 0 | 0 |
| Secondary | ||||
| Male (4) | 2 | 0 | 2 | 0 |
| Female (3) | 3 | 0 | 0 | 0 |
2.1. Surgical Technique
All surgical maneuvers have been applied by one surgeon (AEB) using topical anesthesia (0.4 % Benoxinate hydrochloride: Benox; EGYPTIAN INT. PHARMACEUTICAL INDUSTRIES CO. (E.I.P.I.CO.) – Egypt) and subconjunctival anesthesia (lidocaine 10 mg/ml+epinephrine 0.0125 mg/ml) during the period between October 2104 to March 2015.
As regarding primary Pterygium, the bare scleral technique was performed. After careful dissection, the surgeon excised the Pterygium head with a small part of its body. Furthermore, the underlying tenon tissue was removed from the cornea and the sclera using scissors. Remnant Pterygium tissue on the cornea was separated with a crescent knife. The bare sclera was evaluated with a caliper to leave 2 ml from the limbus. It was essential to secure the conjunctive with 4–6 separated 7/0 vicryl sutures to the surrounding conjunctiva.
For secondary (recurrent) Pterygium, the conjunctiva was freed and settled (but not resect) back to the fornix to prevent conjunctival shortening or symblepharon. Blunt dissection was undertaken to cut the Pterygium head from the cornea, the epithelium of the Pterygium body from the subconjunctival fibrous tissue, and then the subconjunctival fibrous tissue from the sclera. The second step was sealing the gap between the recessed conjunctival edge and Tenon’s capsule by applying a running 9-0 nylon suture to hopefully avoid recurrence.
To manage recurrent Pterygium conditions with limited conjunctival tissue in the caruncle due to the presence of significant conjunctival scarring, a small conjunctival autograft was completed to reconstruct better fornix. The medial rectus muscle was uncovered and hooked after a conjunctival recession, and the conjunctival cicatrix was freed to avoid the muscular injury. As regarding postoperative care, all candidates received an antibiotic eye drop (0.3% Ofloxacin: Exocin; Allergan Inc.) four times a day for 1 week, and lubricant eye drop (Carboxymethylcellulose sodium 0.5% eye lubricant: Refresh Tears; Allergan, Inc., Dupont Drive, Irvine, CA, USA) four times a day for 6 weeks. Corticosteroid eye drop (FLUCON 0.1%; ALCON-COUVREUR – Belgium) was added.
2.2. Laboratory Methods
Pterygium Sample Collection from each patient was sent to Pathology Department Laboratories, Faculty of Medicine, Mansoura University, Egypt in formalin solution 10%. For more accuracy, the samples were labeled by serial ID numbers with full personal and clinical data.
2.3. Sample Processing
The samples were remolded in Paraffin blocks and categorized into two different groups: the Primary Pterygium group (20 samples) and recurrent Pterygium group (7 samples). The samples were fixed in formalin and subjected to H & E staining firstly, then immunohistochemistry for both reduced glutathione and topoisomerase I.
2.4. Immunohistochemistry
The samples were stained depending on the avidin-biotin technique. The tissue was heated to 95 degrees in the PT link of Dako for 30 min for antigen retrieval. 3% hydrogen peroxide solution was loaded for 10 min to aiming to block endogenous peroxidase, then washing them in PBS. Then, an overnight incubation was done with antibody dilution of 1/150 of mouse monoclonal anti-Glutathione S transferase (Cat. No. (4B6) ab170323, abcam). Anti-topoisomerase 1with dilution of 1/50 (SC69-03) (NBP 2-67606, Novus) then washing in PBS. After that, a secondary antibody (1:1000; Invitrogen, Carlsbad, CA, USA) was added for 2 h at room temperature. Three washes with PBS were then applied, slides were mounted with a medium containing DAP. Hematoxylin was used to counter the stain. Absolute alcohol was added to clear the field. The positive control was kidney tissue for Glutathione and thyroid tissue for topoisomerase 1. Negative control slides were subjected to the same step, but no antibody was added.
2.5. Immunohistochemistry Assessment
2.5.1. Percentage of Positive Cells
• Positive staining: Brown staining of ≥5% of the total number of epithelial cells is considered positive for both glutathione (cytoplasmic) and topoisomerase 1 (nuclear).
• Negative staining: if brown cells represent less than 5% of total epithelial cells.
• Mild positive (score 1): if brown stained cells represent 5-25%
• Moderate positive (score 2): if brown stained cells represent 25-50%
• Strong positive (score 3): if brown stained cells represent ›50%
2.5.2. The Intensity of Staining
• Weak positive (score 1): if the intensity of staining is weak (light brown)
• Moderate positive (score 2): if the intensity of staining is a moderate degree.
• Strong positive (score 3): if the intensity of staining is very dark brown
2.5.3. Total Scoring
Total scoring = score of percentage of positive cells + score of the intensity of staining.
Grade 0 (negative) for score 0 or 1
Grade 1 positive for score 2
Grade 2 positive for score 3 or 4
Grade 3 positive for score 5 or 6
Statistics: the study was analyzed by SPSS package version for descriptive statistics.
3. RESULTS
About 74% of the total cases were primary Pterygium; more than 51% of them were males. Also, males dominated the secondary Pterygium of about 67%. Males were dominating the number of Pterygium cases in either primary or secondary Pterygium. The age of patients in the current study ranged from 18 to 55 years.
• Results of Hematoxylin and Eosin staining:
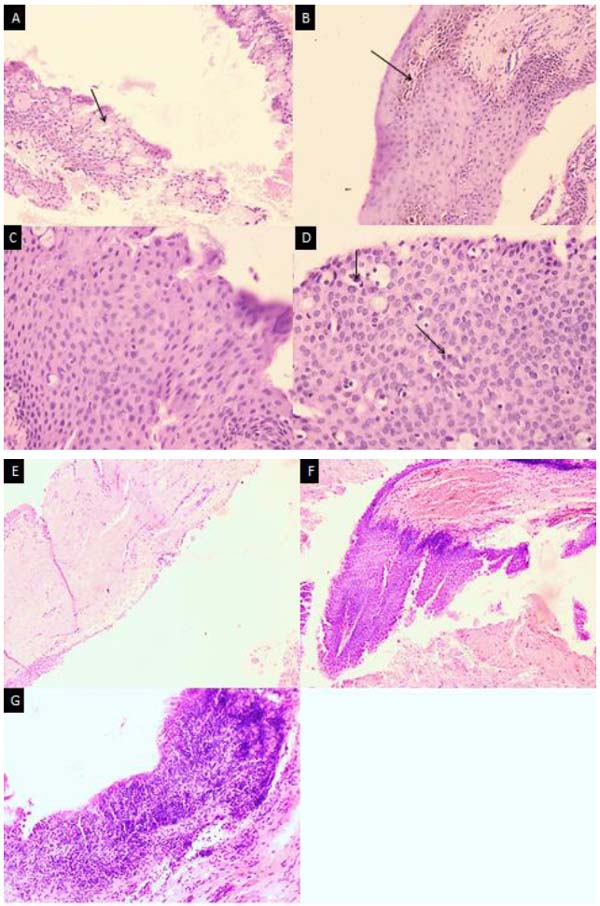
Fig. (1) showed The H and E of both primary and secondary Pterygium. The slides demonstrated epithelial hyperplasia and atypia in the primary forms. The reaction has been more intense in the secondary Pterygium demonstrating ulcerations and more dysplastic activity of the epithelium.
The epithelial changes included epithelial atypia in 40% of cases, mostly reactive atypia mild to a moderate degree equally distributed in primary and secondary Pterygium. Severe dysplasia (2cases) was observed in secondary Pterygium. Epithelial hyperplasia was observed in all cases of primary and secondary Pterygium. Goblet cell hyperplasia was observed in 30% of primary Pterygium cases and 50% of secondary cases. Prominent epithelial pigmentation was present in 30% of cases. Mild epithelial lymphocytic exocytosis was observed in 65% of cases; the moderate form was observed in 35% of cases, mostly secondary (Fig. 1).
Stromal inflammation had a perivascular pattern in 70% of cases. 30% of cases showed no stromal inflammation. Stromal solar elastosis was evident in all cases. Evident stromal vascularity was observed in 50% of cases with higher incidence with secondary cases (Fig. 2).
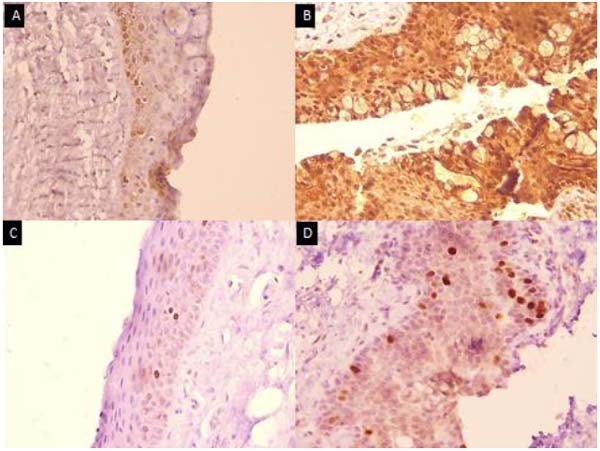
It was recorded that 85% of the cases were positively stained with reduced glutathione. The remaining 4 cases (15%) of negative cases were primary and 3 of them were males. Most (75%) of primary cases of Pterygium were positive for reduced glutathione. More than 60% of them have a high grade of staining; indicating the role of oxidative stress leading to degeneration in the pathogenesis of the disease.
All secondary causes of Pterygium were positive for reduced glutathione; all of them expressed high-grade staining for reduced glutathione representing more release of oxidative stress factors with secondary cases than primary ones.
All cases of Pterygium were negative for topoisomerase 1except two cases (7.4%) were secondary cases with moderate grading of positivity. However, all cases showed internal control stem cells positivity scattered focal between the basal cell layers of covering epithelium.
Fig. (3) showed the immunostained slides for both reduced glutathione and topoisomerase 1for both primary and secondary Pterygium. Primary Pterygium slides were mildly positive for reduced glutathione and only stem cells got positive for topoisomerase 1alpha. On the other hand, secondary Pterygium expressed more glutathione stain and mild positive topoisomerase 1 stain not only for stem cells but also for the superficial prickle cells in the secondary Pterygium
4. DISCUSSION
The current study examined both primary and secondary Pterygium immunohistochemistry staining with both reduced glutathione and Topoisomerase 1. The reduced glutathione immune stain reflected the redox status and the degenerative component of the disease [7, 9]. Lymphangiogenesis has been proposed as a mechanism of Pterygium [10]. There are strong risk factors of oxidative inflammation, viral causes, and oncogenic activation in the contribution of the development of the double hybrid pathology Pterygium. Studies recommended the human papillomavirus as a candidate for being a causing virus of Pterygium [11].
The reduced glutathione immune stain showed a strong reaction in most of the cases with marked stain percentage and intensity in the secondary forms. Other studies supported the idea that oxidative stress induced by ultraviolet rays is part of the pathogenesis of Pterygium. They found a positive defensive reaction of malondialdehyde (MDA) levels, superoxide dismutase (SOD), and catalase (CAT) activities in the conjunctival samples taken from patients suffering from Pterygium and cataract as an aging process [12, 13]. A very close work showed that a very similar glutathione immune staining score in both intensity and spread. The author attributed the positive glutathione results to the defensive antioxidant capacity against free radicals generated by the sun ray’s exposure [14].
Ultraviolet rays have dual degenerative/ proliferative effects that may promote Pterygium. These rays stimulate the VEGF (Vascular Endothelial Growth Factor), promoting lymphangiogenesis. Experimental inhibition of the VEGF prevents the lymphatic reaction [15]. Corneal herpes viral infection may be a contributing factor to the development of lymphangiogenesis as well as ultraviolet factors [16].
The current study noticed that Topoisomerase 1stained the stem cell of the tissue samples reflecting a possible aberrant stem activity. This focal activity may be a marker for progenitor activity to explain the proliferative component of the Pterygium. Topoisomerase 1 may be a clue to the mechanism of recurrence or possibly a prognostic factor. Experimental work on human leukemia cell lines showed increased topoisomerase 1in many cancers like colorectal, ovarian, cervical, prostatic, and even lymphomas [17]. Another important data is that topoisomerase 1over expression was correlated with the bad prognosis of colorectal metastasis [18]. The possible common pathogenesis in both Pterygium and colorectal carcinoma can be explained by K-ras activation that sun rays can mutate. The possible common interaction between both K-ras and topoisomerase alpha may be target pathways for both diseases and even may predict their outcomes [19].
Those slides showed positive staining for Topoisomerases and, at the same time, reduced glutathione are mainly respecting the pattern of secondary behavior. The current study proposes that a panel of immunohistochemistry staining of reduced glutathione, Topoisomerase, and possibly others like p53 or the vascular endothelial growth factor in combination could be a good method to predict the prognosis of Pterygium. Limitations of this study include the small number of cases and the limited number of immune panels like p53 and possibly others like aging markers.
CONCLUSION
Pterygium is a wide world multifactorial disorder. The degenerative component is strongly related to oxidative stress conditions detected by immune stain with glutathione, while the proliferative component can be explained by stem cell activity demonstrated by focal topoisomerase 1 activity. A panel of immunohistochemistry may be a good tool to predict the recurrence rate.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Board of the Faculty of Medicine, Mansoura University, Mansoura, Egypt with code number R.20.01.728.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all the participants.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We are thankful to the technician Ahmed Hashish of the pathology department of Mansoura University.