LETTER ARTICLE
Transepithelial Corneal Cross-linking with Supplemental Oxygen in Keratoconus Treatment - Corneal Stromal Demarcation Line and Safety
Jessica Qian Hui Choo1, Li Lim1, 2, *
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
E-location ID: e187436412207130
Publisher ID: e187436412207130
DOI: 10.2174/18743641-v16-e2207130
Article History:
Received Date: 27/12/2021Revision Received Date: 28/2/2022
Acceptance Date: 31/3/2022
Electronic publication date: 30/08/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose:
To evaluate the corneal stromal demarcation line and safety of transepithelial corneal cross-linking (CXL) with supplemental oxygen in progressive keratoconus treatment.
Methods:
This is a retrospective review of 25 patients with progressive keratoconus who underwent epithelial-on CXL with supplemental oxygen from December 2019 to February 2022. Outcomes measured include corneal stromal demarcation line depth, volume of cornea treated, endothelial cell count, best-corrected visual acuity, keratometric parameters and post-treatment adverse events.
Results:
25 eyes of 25 patients were included and mean age was 28.3 years. Mean follow-up period was 11.5 ± 1.39 months. Pre-operatively, mean ± standard deviation (SD) of K1, K2, Kmax and minimal corneal thickness were 45.9D ± 3.79D, 50.2D ± 4.83D, 57.5D ± 6.98D and 482.3um ± 36.8um respectively. There is no significant difference between pre and post-treatment corneal topographic parameters. There was improvement in BCVA post-treatment. The mean post-treatment corneal stromal demarcation line depth was 367.3 ± 89.8um. The volume of treated cornea including the central corneal epithelial thickness was 73.3 ± 4.39%. There was no reduction in endothelial cell count (ECC) post-procedure (pre-treatment mean ECC±SD: 2695.4 ± 224.5 cells/mm2, post-treatment ECC 2730.1 ± 252.0 cells/mm2, p-value = 0.33). Post-treatment corneal haze was mild and seen in 8 patients postoperatively. One patient developed a non-visual axis involving stromal infiltrate that resolved with topical broad-spectrum anti-microbials.
Conclusion:
Trans-epithelial CXL with supplemental oxygen for keratoconus treatment achieved comparable corneal stromal demarcation line depth comparable to that of conventional epithelial-off corneal cross-linking and had a similar safety profile.
1. INTRODUCTION
Keratoconus is a bilateral progressive corneal ectatic disease with progressive corneal thinning and steepening [1]. Depending on severity of keratoconus, extent of cornea thinning and irregular astigmatism, visual acuity, visual demands, presence of corneal scarring and previous episodes of hydrops, management of keratoconus ranges from surveillance to corneal transplantation. The standard treatment modality of progressive keratoconus is corneal cross-linking (CXL) to limit ectatic progression. In the conventional CXL protocol first described by Wollensak et al [2] in Dresden, the central corneal epithelium is debrided, topical riboflavin applied to the corneal stroma, after which it is irradiated with ultraviolet-A light (UVA) at 3 mW/cm2 for 30 minutes. The concept is that epithelial debridement allows good stromal penetration by the riboflavin, and coupled with UVA photoactivation, singlet oxygen is generated, leading to covalent bond formation [3]. Efficacy of this original protocol has been well-illustrated in ex vivo studies [4] and randomized clinical trials [5, 6].
Epithelium-on CXL has been proposed to reduce potential complications such as pain, delayed epithelial healing or infectious keratitis [7, 8]. However, potential limitations include shallower demarcation line depth, reduced flattening of maximum keratometry and potentially greater risk of keratometry progression [9]. Despite treatment modifications to improve riboflavin permeation, initial attempts at transepithelial CXL have not been as efficacious as the conventional protocol [10, 11]. Shalchi et al. conducted a meta-analysis that demonstrated inconsistent efficacy in epithelial-on studies as compared to epithelial-off studies [12]. 29 out of 45 epithelial-off studies and 5 out of 6 epithelial-on studies reported data on maximum keratometry, of which only 6.9% of the epithelial-off studies showed progression compared to 60% of the epithelial-on studies.
The barrier function of the corneal epithelium may limit the availability of all three key components in CXL, namely riboflavin, UVA and oxygen. It is known that corneal stiffening is reduced when CXL is performed in a low oxygen environment [13] and increased in high oxygen environments [14]. Ex-vivo studies show that oxygen is rapidly consumed during UVA irradiation under atmospheric conditions [15]. Thus, improving oxygen bioavailability during CXL can enhance the corneal stiffening effect and this can be done via pulsed illumination to slow oxygen consumption [16-18] or oxygen supplementation to maintain a high oxygen environment [14].
Matthys et al [19] in France published a study earlier this year evaluating a trans-epithelial CXL procedure combining high irradiance, high-dose, pulsed UVA illumination and supplemental oxygen delivery through Boost Goggles (Glaukos Corporation). Currently, this is the only study in literature with this new CXL protocol. There is a paucity of published literature on the outcomes and safety of transepithelial corneal cross-linking (CXL) with supplemental oxygen. In particular, there is no published data on its effects on Asian eyes to date. In this study, we evaluate the corneal stromal demarcation line and safety of this new technique of transepithelial corneal cross-linking (CXL) with supplemental oxygen in the treatment of progressive keratoconus.
2. MATERIALS AND METHODS
2.1. Study Design
This is a retrospective clinical audit of 25 patients with progressive keratoconus who underwent epithelial-on CXL with supplemental oxygen from December 2019 to February 2022 in Singapore National Eye Centre by a single surgeon (LL). As this study was a clinical audit, it was exempted from review by the Singhealth Centralised Institutional Review Board.
The inclusion criteria for this study were: patients 18 years of age and above, progressive keratoconus (characterized by an increase of 0.50D diopters [D] or higher in maximum keratometry [Kmax] over 1 year), and thinnest corneal thickness of at least 400 μm. The exclusion criteria for this study were: history of corneal surgery or other corneal pathology, aphakic eyes, pseudophakic eyes without a UV-filtering intraocular lens implant, presence of nystagmus, known allergy to any substances used during the CXL procedure, pregnancy and lactation. If the patient was a contact lens wearer, he/she had to stop contact lens wear for 1 week before the initial and subsequent follow-up visits.
2.2. Procedure
Topical anaesthetic (Tetracaine; Thea) was applied to the eye undergoing the procedure. Then, using a surgical spear moistened with 0.25% riboflavin solution with benzalkonium chloride in hydroxypropyl methylcellulose (ParaCel Part 1; Glaukos Corporation), the mucin layer of the tear film was removed (Wek Cell Sponge; Beaver-Visitec). Thereafter, additional drops of 0.25% riboflavin solution were applied every 60 seconds for 4 minutes in total. At the 4-minute mark, the Part 1 formulation was rinsed away from the eye with 0.22% riboflavin solution without benzalkonium chloride (ParaCel Part 2; Glaukos Corporation). For every 30 seconds up to 6 minutes in total, additional drops of the 0.22% riboflavin solution were applied. Using 5ml of balanced salted solution, excess riboflavin was washed away. Oxygen goggles (Boost Goggles; Glaukos Corporation) supplied by a humidified medical grade oxygen source were used to create a flow rate that resulted in an oxygen concentration of 90% or higher within the goggles, so as to provide a high oxygen level at the cornea surface. The oxygen concentration within the goggles was confirmed using an oxygen analyzer (Model 901 Oxygen and Carbon Dioxide Analyzer, Quantek Instruments, Massachusetts USA). The central 9 mm of the cornea was irradiated through the front opening of the goggles with 30 mW/cm2 365-nm UVA, pulsed at 1-second intervals (1 second on, 1 second off) for 11 minutes and 6 seconds using a UVA delivery device (KXL System, Glaukos Corporation). The total UVA dose was 10 J/cm2. As required, balanced salt solution was instilled onto the cornea through the front opening of the goggles to maintain corneal hydration during irradiation, minimally once every 2 minutes. At the end of the procedure, the cornea was rinsed with 5 mL of balanced salt solution, topical antibiotics were applied (cefazolin 125mg in 0.5ml of sterile water, gentamicin 20mg in 0.5ml solution) and a bandage contact lens was inserted.
2.3. Post-treatment Care
Patients were reviewed on post-operative day 1, week 1, month 1, month 3,month 6 and 1 year unless there have been other reasons for earlier follow-up. Post-CXL, patients were instructed to apply to the treated eye: topical moxifloxacin (Vigamox) every 2 hours, preservative-free lubricant eyedrops (Tears Naturale Free) every 1 hour and loteprednol etabonate (Lotemax) 3 times a day. Oral non-steroidal anti-inflammatory medication (Etoricoxib (Arcoxia) 60mg once daily) was given for 1 week. The bandage contact lens was removed on the first postoperative day. After the first week post-treatment, topical moxifloxacin and the lubricant eyedrops were reduced to four times a day. Together with the other eyedrops, the patient was instructed to continue application till the end of the first month post-treatment.
2.4. Outcome Measures
Primary outcomes measures include post-treatment corneal stromal demarcation line depth measured on Optovue (Avanti RTVue XR 100-2, Optovue, Inc., California USA), volume of cornea treated using values obtained from Optovue, pre- and post-treatment endothelial cell count (Konan Specular Microscope NSPC, Konan Medical, Inc., Hyogo Japan) and post-treatment adverse events. Secondary outcome measures include best-corrected visual acuity and corneal topographical measurements pre- and post-treatment (OCULUS Pentacam, OCULUS Inc., Washington USA) at the post-treatment month 3, 6 and year 1 mark.
For grading the demarcation line measurements, two independent graders evaluated the images and only Grade 3 images were included for the corneal demarcation line depth and volume measurements. Demarcation line measurements were scored into 3 grades as per Spadea et al.: (1) demarcation line not identifiable, (2) demarcation line visible but not clearly measurable, (3) clearly visible and measurable demarcation line [20].
3. RESULTS
25 eyes of 25 patients were included (48.0% right eye 52.0% left eye, 32% female 68% male) and mean age was 28.3 ± 1.2 years. Mean follow-up period was 11.5 ± 1.39 months. Table 1 shows the baseline parameters before CXL. Table 2 shows the parameters after CXL. Pre-treatment, best-corrected visual acuity was 0.307 ± 0.390 logMAR. Keratometry readings were 45.9 ± 3.79 D for K1, 50.2 ± 4.83 D for K2 and 57.5 ± 6.98 D for Kmax. Minimal corneal thickness was 482.3 ± 36.8 um and endothelial cell count (ECC) was 2695.4 ± 224.5 cells/mm2. The mean corneal stromal demarcation line depth obtained post-CXL was 367.3 ± 89.8 um and the volume of treated cornea including central corneal epithelial thickness was 73.3 ± 4.39%.
| Number of Eyes: 25 | Mean ± SD |
| Best-corrected visual acuity (LogMAR) | 0.307 ± 0.390 |
| K1 (Dioptres, D) | 45.9 ± 3.79 |
| K2 (Dioptres, D) | 50.2 ± 4.83 |
| Kmax (Dioptres, D) | 57.5 ± 6.98 |
| Minimal corneal thickness (um) | 482.3 ± 36.8 |
| Endothelial cell count, ECC (cells/mm2) | 2695.4 ± 224.5 |
20 out of 25 eyes had post-procedure corneal stromal demarcation line measurements performed. 12 of these eyes (60%) had Grade 3 findings. The rest (40%) had Grade 2 findings and none had Grade 1 findings. Figs. (1, 2) show examples of Grade 3 and 2 demarcation lines respectively.
| Mean ± SD | |
| Corneal stromal demarcation line depth (um) | 367.3 ± 89.8 |
| Volume of treated cornea including central corneal epithelial thickness (%) | 73.3 ± 4.39 |
Post-CXL ECC was measured in 15 out of 25 eyes with a mean of 2730.1 ± 252.0 cells/mm2. There was no significant reduction in ECC post-procedure (p-value = 0.33). Post-treatment corneal haze was mild and affected 8 patients in total (32.0%). It was seen in 5 patients at post-treatment month 1 (POM1), 3 patients at POM3, and 4 patients at POM6. One patient (4.0%) developed a non-visual axis involving stromal infiltrate that resolved with topical broad-spectrum anti-microbials and cessation of topical steroids. No patients had clinically apparent corneal edema or any evidence of endothelial decompensation.
Our secondary outcomes were the changes in BCVA and corneal topography parameters post-procedure. Table 3 illustrates the comparative data between pre-treatment parameters and post-treatment parameters at post-procedure month 3, 6 and year 1. There is no significant difference between pre and post-treatment corneal topographic parameters in terms of K1, K2, Kmax and minimal corneal thickness at any time point. There was a significant improvement in BCVA at post-procedure month 6 (0.307 to 0.154 logMAR, p = 0.02). The 1 year follow-up showed an improvement in the BCVA (0.307 to 0.145 logMAR), although not statistically significant,
| Parameter | Mean ± SD, p-value | |||
|
Pre-procedure (n = 25) |
Post-procedure month 3 | Post-procedure month 6 | Post-procedure year 1 | |
| Best-corrected visual acuity (LogMAR) | 0.307 ± 0.390 | 0.347 ± 0.435 (p = 0.26) (n = 18) |
0.154 ± 0.161 (p = 0.02) (n = 18) |
0.145 ± 0.179 (p = 0.12) (n = 15) |
| K1 (Dioptres, D) | 45.9 ± 3.79 | 45.2 ± 1.96 (p = 0.20) (n = 8) |
44.9 ± 2.14 (p = 0.43) (n = 11) |
44.3 ± 2.24 (p = 0.14) (n = 11) |
| K2 (Dioptres, D) | 50.2 ± 4.83 | 49.9 ± 3.65 (p = 0.34) (n = 8) |
48.8 ± 4.23 (p = 0.49) (n = 11) |
49.7 ± 5.27 (p = 0.33) (n = 11) |
| Kmax (Dioptres, D) | 57.5 ± 6.98 | 55.9 ± 5.84 (p = 0.27) (n = 8) |
55.6 ± 5.85 (p = 0.11) (n = 11) |
57.3 ± 6.72 (p = 0.33) (n = 11) |
| Minimal corneal thickness (um) | 482.3 ± 36.8 | 496.6 ± 39.6 (p = 0.16) (n = 8) |
492.6 ± 42.9 (p = 0.33) (n = 11) |
489.7 ± 33.3 (p = 0.20) (n = 11) |
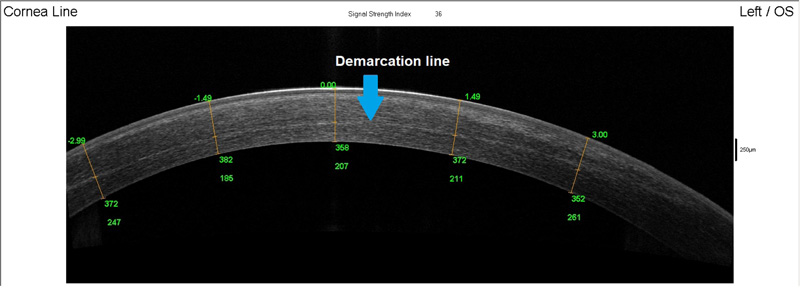 |
Fig. (1). Grade 3 demarcation line at 358um depth. |
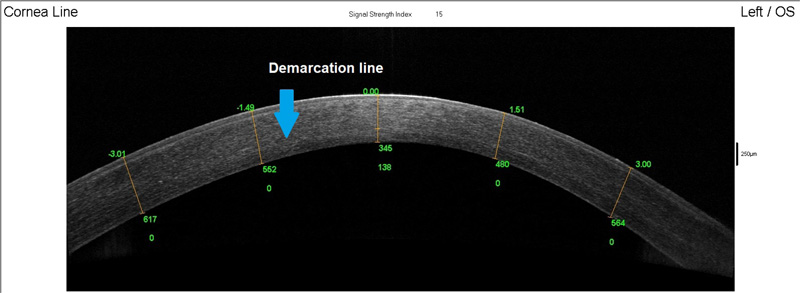 |
Fig. (2). Grade 2 demarcation line at 345um depth. |
4. DISCUSSION
Table 4 compares the post-CXL demarcation line and volume of treated cornea parameters obtained in our study with other studies in current literature. The post-treatment corneal stromal demarcation line depth is generally assumed to be a measurement of treatment efficacy. The demarcation line depth for conventional CXL is typically at about 300um depth [16, 20] and the Epi-on CXL is usually shallower at about 100um depth. The demarcation line depth and volume of treated cornea with this new technique in our study (367.3 ± 89.8 um, 73.3% ± 4.39) is comparable to data published by Matthys et al [19] (Epi-on with O2) and conventional CXL [16, 20] (conventional Epi-off). With supplemental oxygen, greater depth was achieved compared to the Epi-on technique [16]. Our incidence of corneal haze at 32% was less than that reported by Matthys et al. (64.78%) [19] and it was mild. Matthys et al. [19] also reported 11.8% incidence of sterile infiltrate post-treatment attributed to topical NSAID administration. In our study, the 4.0% rate of stromal infiltrate is comparable to the rate of sterile infiltrate typically reported after conventional CXL (from 3.2 to 7.6%) [7, 21] and trans-epithelial CXL (2.4%) [22].
| Parameter | Mean ± SD | ||||||||||
| Study results | Matthys et al. | Spadea et al. |
Maazotta et al. |
||||||||
| Epi-on pulsed with O2 | Epi-on pulsed with O2 | C epi-off | A epi-off | Epi-on | Epi-on iontophoresis | C epi-off | A epi-off | A pulsed epi-off | C Epi-on | A Epi-on | |
|
UVA Energy settings (J/cm2) UVA Power settings (mW/cm2) |
10 30 |
10 30 |
5.4 3.0 |
5.4 10 |
5.4 3.0 |
10 | 5.4 3.0 |
5.4 30 |
5.4 30 |
5.4 3.0 |
5.4 45 |
| Corneal stromal demarcation line depth (um) | 367.3 ± 89.8 | 316 ± 63 | 275.05 ± 41.80 | 279.34 ± 33.06 | 132.60 ± 22.14 | 235.40 ± 37.08 | 350 ± 20 | 200 ± 20 | 250 ± 20 | 100 ± 20 | 100 ± 20 |
| Volume of treated cornea including central corneal epithelial thickness (%) | 73.3 ± 4.39 | 63.34 ± 8.55 | 59.62 ± 6.66 | 34.41 ± 7.50 | 57.58 ± 4.96 | ||||||
Shalchi et al. published a systemic review on epithelium-off CXL versus trans-epithelial CXL without supplemental oxygen for keratoconus [12]. However, there was a lack of suitable trans-epithelial CXL studies included – only 6 studies were considered for this systemic review and none of them utilized supplemental oxygen. Also, it did not include data on corneal stromal demarcation line. In terms of treatment safety, the median percentage of eyes in epithelium-off CXL studies with stromal haze was 9.8% (range 0 – 100%) versus 0% (range: 0 – 4%) in trans-epithelial CXL studies (12 out of 45 epithelium-off CXL studies and 4 out of 6 trans-epithelial CXL studies reported on stromal haze). The epithelium-off CXL studies included which reported sterile infiltrate rates were at median percentage of 2.5% (range between 2-4%, out of 6 epithelium-off CXL studies), whereas none of the trans-epithelial CXL studies included reported on sterile infiltrate rates. With regards to incidence of microbial keratitis, the median percentage was 0% in epithelium-off CXL studies (range 0 – 3% in 7 studies) and 0% in all 4 trans-epithelial CXL studies that reported microbial keratitis incidence. For changes in ECC, there was an included randomized clinical trial that did not show any significant difference after epithelial-off or trans-epithelial CXL studies.
With reference to comparing changes in BCVA and corneal topographical parameters to findings obtained in other studies, epithelial-off CXL tended to show a significant improvement in BCVA and Kmax [2, 5, 6]. Our study showed a similar improvement in BCVA post-CXL. However, there was no difference in pre-CXL and post-CXL K1, K2 and Kmax parameters. This shows that although improvement in keratometry parameters was not seen, there was no post-procedure regression, indicating that this procedure is effective in stabilising and preventing further worsening of keratoconus. In contrast, epithelial-on CXL without supplemental oxygen has shown post-procedure keratometric regression and worsened thinnest corneal thickness [9, 11]. To add on, Shalchi’s review article found that epithelial-on CXL studies in current literature were variable in outcomes in terms of Kmax at 1 year and thinnest corneal thickness, with some reporting reduction in these values post-procedure, whereas some reported increase in these values post-procedure [12]. Shalchi also summarized a median improvement of BCVA by -0.07 logMAR (range: -0.12 to -0.04 logMAR) at 1 year in applicable epithelial-on CXL studies [12]. This is smaller than the BCVA improvement we saw in our study when utilizing CXL with supplemental oxygen. In terms of comparing changes in BCVA and corneal topographical parameters for epithelial-on CXL with supplemental oxygen, Matthys did show statistically significant decreased Kmax, K1, K2 and BCVA but there was also a higher rate of corneal haze seen in his study population [19]. This may indicate that depending on different patient populations, CXL settings may have to be individualized to optimize outcomes.
The limitation of our study is that it is a retrospective study with a small sample size. Post-CXL data was also incomplete for certain parameters in this retrospective study due to patients defaulting or refusal of certain tests due to cost reasons. This was a reason why not all patients had post-CXL demarcation line depth measurements. Also, although the post-procedure ECC data is incomplete, it is reassuring that none of these patients had corneal edema or decompensation, and also there was no reduction in BCVA. In fact, there was improvement in VA post-procedure at 6 months (significant) and 1 year follow-up (insignificant). Further prospective studies are required to refine the treatment parameters and to validate the safety and efficacy of the procedure.
CONCLUSION
Trans-epithelial CXL with supplemental oxygen for keratoconus treatment achieved comparable corneal stromal demarcation line depth to that of conventional epithelial-off corneal cross-linking and had a similar safety profile. Comparing pre and post-CXL, there was no keratometric regression and mean minimal corneal thickness remained stable. There was improvement in BCVA post-procedure.
LIST OF ABBREVIATIONS
| CXL | = Corneal Cross-Linking |
| SD | = Standard Deviation; |
| ECC | = Endothelial Cell Count |
| POM | = Post-operative Month |
| UVA | = Ultraviolet-A |
| IRB | = Institutional Review Board |
AUTHORS' CONTRIBUTIONS
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lim Li and Jessica Choo. The first draft of the manuscript was written by Jessica Choo and all authors commented on previous versions of the manuscript. Writing, review and editing was performed by Lim Li. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was a clinical audit. As such it was exempted from review by the Singhealth Centralised Institutional Review Board.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans used were in accordance with the the Singhealth Centralised Institutional Review Board And with the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
As this was a clinical audit, there was no informed consent obtained from the patients. However, there is no identifying information included in the manuscript.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
As this is a clinical audit study, the datasets generated during and analysed during the study are not publicly available.
FUNDING
No funding or sponsorship was received for this study. The publication fee will be funded by Glaukos.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGMENTS
Declared none.
DISCLOSURE
Choo reports no conflicts of interest in this work. Lim received speaker fees from Glaukos, Santen and Johnson&Johnson, and travel funds from Glaukos and Santen. She also has a research grant with Santen.
PRIOR PRESENTATION
Presented as a free virtual paper in the 39th congress of the ESCRS(Amsterdam) on 9th oct 2021,Session: keratoconus







