All published articles of this journal are available on ScienceDirect.
Comparison of Clinical Results, Contrast Sensitivity and Optical Quality Following the Implantation of CT LUCIA 611P and TECNIS-1 ZCB00 MonofocalIOLs -12 Month Outcomes
Abstract
Purpose:
To compare the clinical performance, contrast sensitivity and optical quality, following implantation of CT LUCIA 611 P and TECNIS-1 monofocal IOLs following cataract surgery.
Design-Prospective, interventional, non-randomized comparative study.
Setting- Nethradhama Super Speciality Eye Hospital, Bangalore, India.
Methods:
Eligible patients, undergoing phacoemulsification received implantation with CT LUCIA 611P or TECNIS-One piece (TECNIS-1) monofocal IOLs.
Results:
Hundred eyes from 100 patients were sequentially divided into CT LUCIA and TECNIS-1 groups, with 50 eyes in each group.Intra-operatively, the mean unfolding time was significantly longer (35.16 ± 10.50 sec) in the TECNIS-1, compared to the CT LUCIA group (12.93 ± 3.80 sec), p= 0.00.At 12 months, 80% (40) eyes in the CT LUCIA and 76%(38) eyes in the TECNIS-1 group had cumulative UDVA of 20/20 or better. No significant differences were found between the mean values of post-op UDVA, CDVA, contrast sensitivity (all spatial frequencies), Objective Scatter Index (OSI), and Modular Transfer Function (MTF) between both groups. A significantly higher value of internal coma and SA for the Tecnis-1 IOL group was noted (p<0.05). However, there was no significant difference between the total HOA, coma and SA for both the groups. Six eyes in the TECNIS-1 group had intra-operative adhesions of the haptics with optic / haptic, requiring additional manipulation.
Conclusion:
At one year, both monofocal IOLs delivered comparable clinical outcomes.However, CT LUCIA 611P IOL had significantly less internal coma and SA, unfolding time and smoother IOL insertion without any issues due to poor loading.
1. INTRODUCTION
While there is no ideal monofocal intraocular lens (IOL) available as yet, significant improvements in the field of monofocal IOL technology have occurred in the past few decades to achieve an IOL design as close to perfection. A novel IOL design must offer excellent optical performance without unwanted side effects, should be made using a high-purity material to reduce the incidence of glistening, has good capsular and uveal biocompatibility, possess optics that account for eyes requiring aspheric, spheric or neutral corrections and feature a design that fits through small corneal incisions to allow for optimal centration in the capsular bag [1-3].Apart from these, the lens should include square edge technology to prevent posterior capsule opacification (PCO) [4, 5].
A new monofocal IOL is thus expected to fulfil all these properties. In 2016, a hydrophobic acrylic, heparin-coated, fully pre-loaded monofocal IOL,CT LUCIA 611P IOL(Carl Zeiss Meditec, Jena, Germany) was introduced as a successor of CT LUCIA 601 with certain modifications in the design of its optic-haptic junction for better capsular bag stability [6].
In the present study, we compared the 12-month post-operative visual outcomes, refractive stability, aberrations and rate of posterior capsule opacification (PCO) formation following implantation of CT LUCIA 611 IOL with TECNIS-1 ZCB00 IOL; the safety, efficacy and optical quality of which has already been established in various clinical studies [7-9].We also wanted to verify, if the heparin coating on the CT LUCIA 611 IOL, lead to an increased scatter or degradation of optical quality. Hence, Objective Scatter Index (OSI), Modular Transfer Function (MTF)and contrast sensitivity were also compared between the two IOLs.
2. MATERIALS AND METHODS
This prospective study was approved by the institutional ethics committee of Nethradhama Eye Hospital, Bangalore, and adhered to the tenets of the declaration of Helsinki. All patients provided written informed consent. A total of 100 eyes from 100 patients were included in the study and divided into two groups: CT LUCIA and TECNIS-1, with 50 eyes in each group.The first group of 50 patients who met the enrolment criteria received CT LUCIA, while the next group of 50 patients received the TECNIS-1 monofocal IOL.
Inclusion criteria were healthy eyes besides senile cataract NC 1-2 (LOCS III grading); corneal astigmatism equal to or less than 0.75 dioptres (D); IOL powers between +10.00 D and +32.00 D, in the capsular bag IOL implantation. Exclusion criteria were eyes with irregular astigmatism, corneal dystrophy, pupillary abnormalities, history of glaucoma or intraocular inflammation, macular disease or retinopathy, neuro-ophthalmic diseases, and intraoperative or postoperative complications.
Before the surgery, all patients underwent complete ophthalmologic examination including manifest refraction, slit lamp biomicroscopy, and noncontact tonometry (Tomey NCT, NishiKu, Nagoya, Japan), macular OCT (Optovue, Fremont, USA) and dilated fundus examination.
Biometric assessments were performed using the swept source OCT based optical biometer, IOL Master-700, using Barrett’s Total Keratometry (TK) formula [10]. All eyes were targeted at emmetropia. The optimized A- constants of 119.9 and 119.15 were used for the CT LUCIA 611 P and TECNIS-1 IOLs respectively.
2.1. Description of Study IOLs
Table 1 shows the comparison of specifications of CT LUCIA 611Pand TECNIS-1 monofocal IOLs.
The CT LUCIA 611P (Carl Zeiss Meditec, Jena, Germany) is a fully preloaded hydrophobic, acrylic, single-piece, heparin-coated IOL with an overall diameter of 13 mm and an optic diameter of 6 mm. The lens is available in clear UV-blocking (611P) and with blue light filtering (yellow tinted as 611Y) in a range of 4.00–34.00 D in 0.50 D increments. It is completely preloaded in the Zeiss Blueject injector to facilitate a fast and easy lens preparation. The 611P is equipped with a 360° square edge design on the entire IOL, including the optic, the haptics and the optic–haptic transition to prevent PCO. In addition, the haptics is step-vaulted to translate the optic posteriorly for direct contact with the capsular bag. The optic of the lens has a special patented aspheric design, called ZO optic, to compensate for the range of aberrations that arise from different corneal shapes and lens misalignments.
The Tecnis 1-piece IOL(Johnson & Johnson, New Jersey, USA) has a 6.0 mm biconvex optic, an overall diameter of 13.0 mm, and an anterior aspheric surface. The optic has a continuous 360-degree square frosted edge. The C-shaped haptics are offset from the optic for 3-point fixation. The optic and haptics are of an ultraviolet light–filtering hydrophobic acrylic material.
| Feature | CT Lucia 611 P | Tecnis-1 |
| Optic design | Monofocal aspheric | Monofocal |
| Shape | Biconvex, anterior aspheric surface, square optic edge | Biconvex, anterior aspheric surface, square optic edge |
| Material | Hydrophobic acrylic with heparin coated surface and blue light filter | UV blocking hydrophobic acrylic |
| Refractive index | 1.49 | 1.47 |
| Optic diameter | 6.0 mm | 6.0 mm |
| Total diameter | 13.0 mm | 13.0 mm |
| Haptic | Step vaulted | Haptic offset from optic |
| Lens design | Single piece | Single piece |
| Incision size | 2.2-2.6mm | 2.2-2.4mm |
| Company labelled A constant | 119.9 | 119.3 (Optical biometry) 118.8 (Ultrasound biometry- Contact) |
| Dioptre range | +4.0 to +34.0, 0.5 D increments |
+5.0 to +34.0, 0.5 D increments |
| ACD | 6.14 | |
| Abbe number | 50 | 55 |
| Implantation in | Capsular bag | Capsular bag |
2.2. Surgical Procedure
All surgeries were performed by a single experienced surgeon (S.G.), using a standard phacoemulsification technique under topical anaesthesia. All surgeries in both groups were performed from the temporal site, through a clear corneal incision of 2.8 mm size, using the Centurion Precision system (Alcon). A standard capsulorhexis of between 5.0 -5.5 mm was aimed and a direct chop technique was used for nuclear deployment. In the CT LUCIA group, the IOL injection was facilitated through the fully pre-loaded Zeiss Blueject injector, while in the TECNIS-1 group, the UNFOLDER Platinum 1 Series Screw-Style Inserter (Johnson & Johnson) was used to inject the TECNIS-1 IOL through a 2.8 mm temporal clear corneal incision. IOL insertion was done under cover of OVD.
Intra-operative unfolding time was recorded by an independent observer (S.B.) using a stopwatch. Time was documented beginning from when the trailing haptic folded on the optic released from the injector, and stopping when both the IOL haptics completely unfolded inside the capsular bag. Note of any intra-operative complications or difficulty with IOL injection was made.
Post-operative topical therapy included topical prednisolone (1%, Pred Forte, Allergan), moxifloxacin (0.5%, Vigamox, Alcon), and nepafenac (0.1%, Nevanac, Alcon).
Post-operative follow-up examinations were performed at 1 day, 2 weeks, 6 weeks, 6 months, and 12 months. The following tests were performed at all post operative visits from the first week onwards: Slit lamp examination for corneal clarity, anterior chamber inflammation, IOL position, posterior capsule opacification (PCO), measurement of uniocular uncorrected (UDVA) and corrected distance visual acuity (CDVA) using ETDRS charts (Precision Vision, La Sella, IL, USA), uniocular uncorrected (UNVA), near corrected near visual acuity (CNVA); and mesopic contrast sensitivity (F.A.C.T. Stereo Optical Co. Inc., Chicago) with distance correction. Internal and total aberrations were measured using ray tracing aberrometry (I-trace, Hoya, Japan). A double pass system (HD Analyzer,Visiometrics, Spain) was used to evaluate the OSI and MTF cut-offs. This diagnostic tool measures both the scattered light and higher order aberrations, thus enabling objective assessment of optical quality. All measurements were recorded 5 minutes after instillation of a lubricant eye drop [Lacryl Ultra (polyethylene glycol 0.4%, propylene glycol 0.3%), ENTOD pharmaceuticals] as the dry-eye disease can degrade the quality of retinal image, resulting in higher OSI values.
2.3. Statistical Analysis
SPSS software for Windows version 17.0.0 (IBM Corp., Armonk, NY) was used for statistical analysis. All values were expressed as mean ± standard deviation (SD). Data was checked for normality before subjecting to analysis. If the data was normally distributed, then the Independent sample t-test was used for intergroup comparison and paired t-test was used for intra-group comparison of means. If the data distribution was not normal, Wilcoxon signed-rank test was used. A p-value of 0.05 or less was considered statistically significant. Outcome analysis was performed according to the Standard Graphs for Reporting Refractive Outcomes Intraocular Lens-Based Refractive Surgery [11].
3. RESULTS
Table 2 shows the pre-operative characteristics and demographic data of both the study groups.
|
Parameter (mean ±SD) Range [min, max] |
CT Lucia 611 P (n=50) |
Tecnis-1 (n=50) |
p-value |
| Pre-operative | |||
| Age(years) | 67.81± 8.9 [45.79] |
67.79± 5.7 [60.80] |
0.09 |
| AL(mm) | 23.80 ± 1.21 [22.13, 27.95] |
23.76 ± 1.13 [21.9, 26.5] |
0.86 |
| K1(D) | 43.64 ± 1.80 [49.92, 47.94] |
43.82 ± 1.20 [41.62, 46.45] |
0.56 |
| K2(D) | 44.16 ± 1.83 [41.39, 48.68] |
44.28 ± 1.18 [42.23, 46.7] |
0.69 |
| Ast (D) | 0.48± 0.26 [0.13, 0.75] |
0.46± 0.20 [0, 0.75] |
0.56 |
| ACD(mm) | 3.22 ± 0.45 [2.22, 4.72] |
3.16 ± 0.41 [2.1, 4.42] |
0.45 |
| WTW(mm) | 12.01 ± 0.45 [11, 13.1] |
11.98 ± 0.41 [11.2, 12.9] |
0.78 |
| IOL power(D) | 20.47 ± 2.93 [9.5, 24] |
20.00 ± 3.21 [11.5, 24.5] |
0.44 |
| Intra-operative | |||
| Un-folding time (secs) | 12.93± 3.80 [4.53, 26.25] |
35.16 ± 10.50 [14.24, 56.8] |
<0.05 |
Both groups were matched in terms of mean age, axial length, K1, K2, corneal astigmatism, anterior chamber depth (ACD), white to white (WTW) diameter and IOL powers implanted(p-values >0.05, for all parameters).
3.1. Post-operative AC Reaction
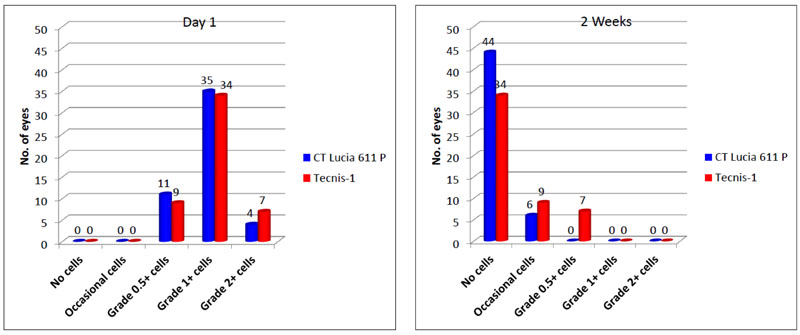
Both groups showed comparable results in terms of postoperative anterior chamber reaction on both days 1 and 2 weeks. No eye in either group had excessive inflammation of more than 2+ cells on post-op day 1 and more than 1+ cells at 2 weeks, (Fig. 1). At 6 weeks post-op, anterior chamber in all eyes were quiet and did not show any evidence of cells or flare.
3.2. Visual outcomes
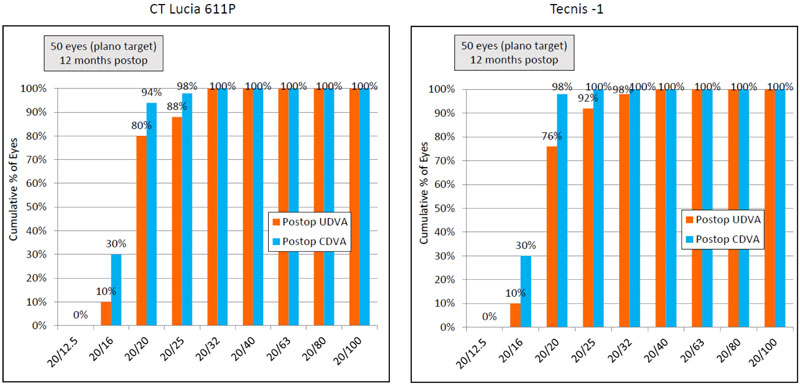
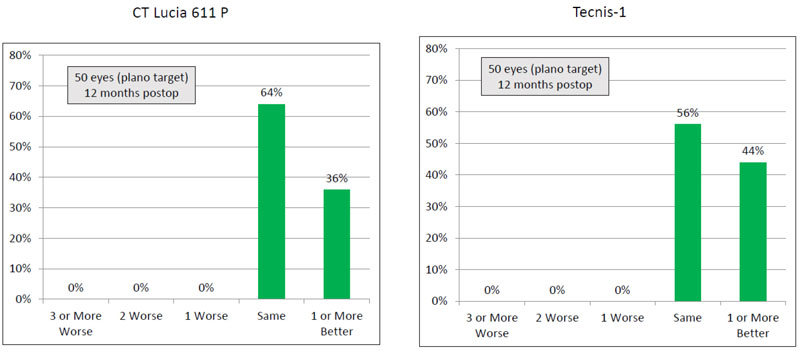
Thirty six percent (18) eyes in the CT LUCIA group had post-operative CDVA one line or better than the post-operative UDVA, whereas this percentage for the Tecnis group was 44% (22 eyes) (Table 3) (Figs. 2 and 3).
3.3. Refractive outcomes
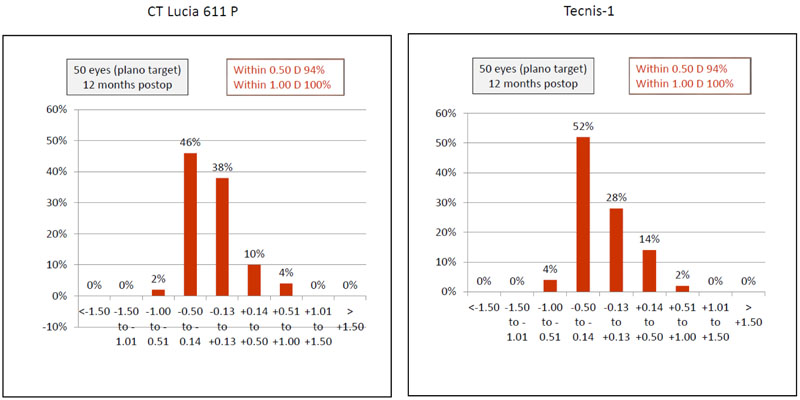
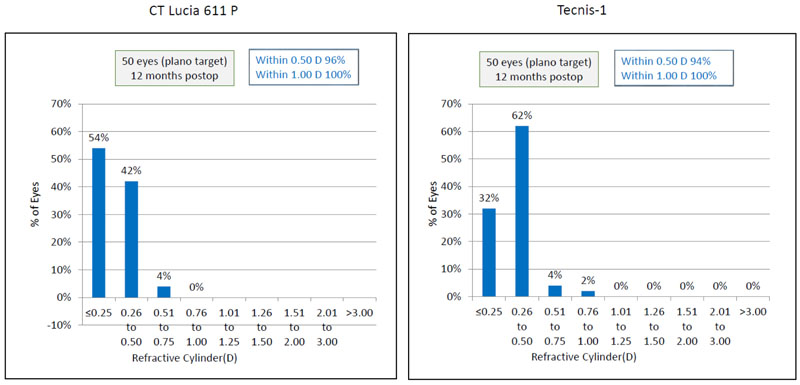
Ninety four percent (n=47) eyes in both groups had SE accuracy within ±0.50 D and all eyes had SE accuracy within ±1.00 D, (Fig. 4).
Post-operative astigmatism was within ±1.00 D in both the groups, (Fig. 5).
3.4. Contrast Sensitivity
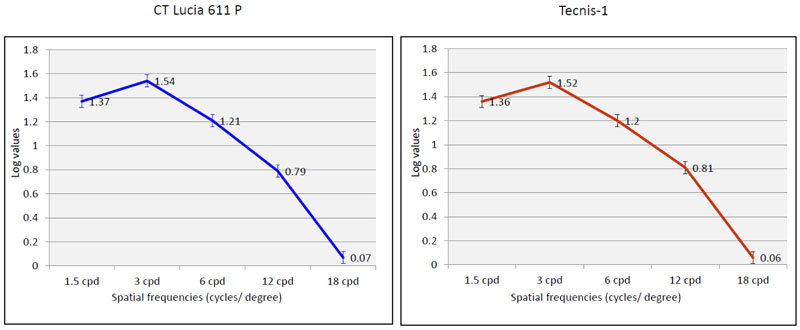
The results of the mean contrast sensitivity values for both the monofocal IOL groups, measured after correction, using the F.A.C.T. chart in photopic conditions, 12 months post-operatively did not show a significant difference for any of the spatial frequencies evaluated (p>0.05, for all spatial frequencies) (Table 2) (Fig. 6).
3.5. OSI and MTF
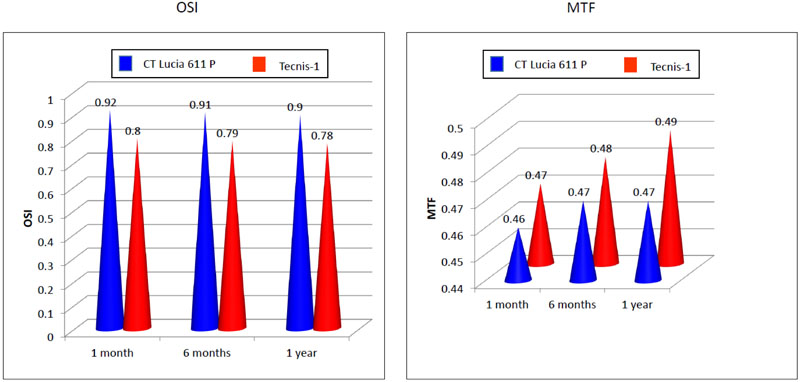
OSI and MTF remained stable through 12 months, with no significant change compared to the 1-month values (One -way ANOVA, p > 0.05 in both the groups), Table 3. At 12 months, the mean OSI values of the CT LUCIA group was 0.90± 0.45 and in the TECNIS-1 group was0.78± 0.45,which was not statistically significantly different. Similarly, there was no significant difference between the mean MTF for the CT LUCIA group (0.47± 0.14)and the TECNIS-1 group (0.49± 0.12) at the last visit (Fig. 7).
3.6. Higher Order & Internal Aberrations
Higher order aberrations evaluated at 4 mm scan size using I-trace, showed a significantly higher value of internal coma (p=0.05) and SA for the TECNIS-1 IOL group (p=0.01). However, there was no significant difference between the mean RMS of internal HOA for both groups. For the total HOA, coma and SA, all values between both the groups were comparable (Table 4).
|
Parameters (Mean± SD) Range [min, max] |
1 month | 6 months | 1 year | p-value |
|
CT Lucia 611 P |
||||
| UDVA(LogMAR) | 0.028± 0.08 [-0.1, 0.2] |
0.022± 0.08 [-0.1, 0.2] |
0.022± 0.08 [0, 0.2] |
0.99 |
| CDVA(LogMAR) | -0.020± 0.06 [-0.1, 0.2] |
-0.022± 0.06 [-0.1, 0.2] |
-0.022± 0.06 [-0.1, 0.2] |
0.98 |
| Sph (D) | -0.065± 0.38 [-0.5, 0.5] |
-0.06± 0.35 [-0.5, 0.5] |
-0.06± 0.35 [-0.5, 0.5] |
0.96 |
| Cyl (D) | -0.11± 0.36 [-0.5, 0.75] |
-0.11± 0.35 [-0.5, 0.75] |
-0.11± 0.35 [-0.5, 0.75] |
0.99 |
| SE(D) | -0.12± 0.31 [-0.75, 0.75] |
-0.11± 0.29 [-0.75, 0.75] |
-0.11± 0.29 [-0.25, 0.5] |
0.97 |
| ACD | 5.05± 0.49 [3.33, 5.75] |
5.01± 0.44 [3.66, 5.7] |
4.98± 0.50 [3.25, 5.68] |
0.71 |
| HD Analyzer | ||||
| OSI | 0.92± 0.46 [0.3, 2.3] |
0.91± 0.45 [0.3, 2.3] |
0.90± 0.45 [0.3, 2.3] |
0.99 |
| MTF | 0.46± 0.13 [0.163, 0.7] |
0.47± 0.13 [0.163, 0.7] |
0.47± 0.14 [0.163, 0.7] |
0.89 |
|
Tecnis-1 |
||||
| UDVA(LogMAR) | 0.034± 0.08 [-0.1, 0.3] |
0.026± 0.08 [-0.1, 0.3] |
0.026± 0.08 [-0.1, 0.3] |
0.83 |
| CDVA(LogMAR) | -0.028± 0.04 [-0.1, 0.1] |
-0.028± 0.04 [-0.1, 0.1] |
-0.028± 0.04 [-0.1, 0.1] |
0.97 |
| Sph (D) | -0.02± 0.16 [-0.5, 0.5] |
-0.03± 0.32 [-0.5, 0.5] |
-0.03± 0.32 [-0.5, 0.5] |
0.93 |
| Cyl (D) | -0.12± 0.44 [-1, 0.75] |
-0.12± 0.43 [-1, 0.75] |
-0.12± 0.43 [-1, 0.5] |
0.99 |
| SE(D) | -0.11± 0.29 [-0.75, 0.75] |
-0.12± 0.27 [-0.75, 0.75] |
-0.12± 0.27 [-0.75, 0.75] |
0.98 |
| ACD | 5.03± 0.48 [3.33, 5.75] |
4.87± 0.41 [4, 5.8] |
4.83± 0.43 [4, 5.8] |
0.58 |
| HD Analyzer | ||||
| OSI | 0.80± 0.46 [0.3, 2.3] |
0.79± 0.46 [0.3, 2.4] |
0.78± 0.45 [0.3, 2.3] |
0.98 |
| MTF | 0.47± 0.17 [0.189, 0.7] |
0.48± 0.12 [0.189, 0.7] |
0.49± 0.12 [0.189, 0.7] |
0.72 |
|
Parameters (µ) (mean ± SD) Range [min, max] |
CT Lucia 611 P (n=50) |
Tecnis-1 (n=50) |
p-value |
| Internal | |||
| HO Total | 0.161± 0.072 [0.019, 0.352] |
0.180± 0.191 [0.034, 1.95] |
0.51 |
| Coma | 0.045± 0.043 [0.01, 0.2] |
0.060± 0.050 [0.012, 0.2] |
0.05 |
| SA | -0.050± 0.063 [[-0.14, 0.045] |
-0.080± 0.054 [-0.132, 0.04] |
0.01 |
| Total | |||
| HO Total | 0.203± 0.115 [0.05, 0.43] |
0.168± 0.080 [0.054, 0.447] |
0.08 |
| Coma | 0.043± 0.100 [-0.126, 0.242] |
0.071± 0.049 [0.011, 0.362] |
0.10 |
| SA | 0.029± 0.047 [-0.06, 0.09] |
0.026± 0.021 [-0.029, 0.101] |
0.75 |
3.7. Intra-operative unfolding time and adverse events
The mean intra-operative unfolding time was significantly higher (35.16 ± 10.50) in the TECNIS-1 group, compared to the CT LUCIA group (12.93 ± 3.80), p= 0.00 Six eyes in the TECNIS-1 group had intra-operative adhesions of the haptics with optic / opposite haptic, requiring additional manipulation to release the same with a second instrument. However, no eye in either group had an occurrence of trapped trailing haptic, overriding of the plunger over the optic, trauma to the optic edge or breakage of haptics. All procedures in both groups were otherwise uneventful. No post-operative complications such as cystoid macular oedema, post-op uveitis, secondary glaucoma or posterior capsule opacification requiring YAG- Capsulotomy were noted in any of the eyes of either group. No IOL in either of the study group required to exchange or explantation due to any reason.
4. DISCUSSION
Recently, Borkenstein et al. evaluated one-year clinical outcomes of a novel designed hydrophobic, acrylic, monofocal, and fully preloaded CT LUCIA 611P intraocular lens.6The optic–haptic junction (Achilles Heel) of the intraocular lens (studied with scanning electron microscopy)was found to be thicker, wider and stiffer than the predecessor (601), with a 360° square edge technology incorporated on it. The post-operative SE was within ±0.50 D in 91.7% and within ±0.75 D in 96.9% of cases. From the surgeons’ perspective, the wider and thicker optic–haptic transition, resulted in increased stiffness, enabling improved centration, refractive predictability, and stability of the IOL [6].In another study, they demonstrated that the CT LUCIA 611 P IOL resulted in good surgical performance, excellent refractive stability, and predictable outcomes in patients with PXF syndrome, phacodonesis and small pupils, where the stability of the capsular bag is already compromised [12].
In the present study, in terms of stability, both CT LUCIA 611 P and TECNIS-1 IOL showed similar stability in the capsular bag. The post-operative ACD remained stable, and no significant myopic or hyperopic shift was observed at 12 months, compared to 1 month in either of the groups. The newly designed thicker and stiffer optic–haptic junction thus appeared to improve the stability of the CT LUCIA IOL in the capsular bag, preventing any significant refractive changes in the long term.
Optical quality evaluated with HD Analyzer did not show any significant difference between the mean OSI and MTF values between both the lenses, at any post-operative time point. No eye in either group showed any evidence of PCO at 12 months due to the continuous 360 degrees square edge, effectively preventing LEC migration [7]. It may be inferred, that in the absence of PCO, the heparin-coated surface of the CT LUCIA IOL, did not result in increased scatter, and subsequent increase in the OSI values. The high and comparable MTF values of both the IOLs may be indicative of an absence of glistening due to the excellent quality of the material used [6, 13].
The perceived benefits of pre-loaded injectable IOL delivery systems include the elimination of the IOL injector loading variability seen in non-preloaded systems, avoidance of potential loading error, and reduced surgical time [14].Intraoperative problems with acrylic intraocular lens (IOL) insertion and post-operative implications due to this have been previously reported [15].Improper unfolding caused by one of the haptics sticking to the optic is known to occur due to inadequate OVD in the cartridge or rarely by the incorrect loading of the IOL [16].In a study evaluating the delivery characteristics of the AcrySof IQ SN60WS intraocular lens (IOL) injected via a preloaded AcrySert delivery system, 47 of the 85 eyes (55%) required additional rotational manipulation, management of trapped trailing haptic, haptic-optic adhesion, overriding of the plunger over the optic, and trauma to the optic edge [17].On the other hand, the fully preloaded feature of the CT LUCIA611 IOL was clearly seen as an advantage in the present study, as all implantations with CT LUCIA 611 IOL were smooth and problem free.
It is known that the mechanical properties of most polymers, including acrylics, are affected by the temperature, and the glass-transition temperature (Tg) of the polymer determines the ideal temperature for optimal unfolding within the eye [18].Chung et al.compared the characteristics of 5 different pre-loaded and non-preloaded intraocular lens (IOL) delivery, systems and found that the average time for non-preloaded systems was comparatively higher than the pre-loaded ones. MX60 had the highest IOL unfolding time in the capsular bag, due to its high “Tg” [19]. The glass transition temperature of TECNIS-1 IOL being comparatively higher (13.8° C) than CT LUCIA (11-12° C), this may explain the significantly shorter unfolding time of the latter, in the present study. In addition, the Heparin Surface Modification (HSM) provided on the CT LUCIA 1OL may also play a role in its faster unfolding.
In terms of the post-operative higher order aberrations, the internal coma and SA (i.e. arising from the internal optics) were found to be significantly higher in the TECNIS-1 group. This could suggest a higher degree of IOL tilt inside the capsular bag in this group. The internal spherical aberrations are expected to be higher with the TECNIS-1 IOL, due to the relatively higher negative SA (-0.27 μm) compared to the CT LUCIA 611P IOL (-0.12μm) [6, 7].It has been proposed that the CT LUCIA 611P IOL is equipped with ZO(600) aspheric concept optic, which means that the power of the IOL is higher in the centre and varies towards the periphery. This results in a flatter lens surface at an intermediate distance from the lens axis and a steepening at the peripheral region of the lens, which may potentially compensate for different corneal shapes and reduce the incidence of higher-order aberrations (HOAs). This should also make the lens less sensitive to decentration and tilt [6]. The ZO aspheric optic, combined with the optic-haptic modification, maythus explain the significantly lesser internal coma seen in the CT LUCIA group, suggestive of better centration in the capsular bag.
TheTECNIS-1IOL with more negative SA (IOL power decreases from centre to periphery) is designed to compensate for corneal aberrations and improve contrast sensitivity [7]. However, this doesn’t appear to have conveyed any added benefit, as the total SA was similar in both groups. As postulated earlier, lenses with higher negative asphericity, if not well aligned, may show increased HOAs such as coma, potentially degrading the visual quality [19-21].In our study, although the Tecnis-1 group had significantly higher internal coma, it did not seem to clinically affect the visual quality, as finally it is the total (whole eye) aberrations that matter.In the study, we did not evaluate corneal aberrations pre-operatively, as the main aim was to determine post-op internal aberrations (indirectly suggesting IOL position) and total aberrations (sum of corneal and internal aberrations) which determine the overall visual quality. It may be possible that the internal coma was compensated by the corneal aberrations in this group, and did not lead to visual degradation.
Heparin Surface Modification (HSM) of hydrophobic acrylic IOLs was suggested as a means to reduce post-operative inflammation [22].However, our study, did not find any clinically significant difference in the post-operative inflammation between both the groups. This may be due to the reason that we included only eyes with no other ocular co-morbidity besides age related cataracts such as pseudo exfoliation, chronic uveitis etc.The HSM of the CTLUCIA 611 IOL, however, did not seem to affect the optical quality,which was evident from comparable values of OSI, MTF and contrast sensitivity between the two groups at 12 months post-operatively.
CONCLUSION
In conclusion, both monofocal IOLs delivered excellent and comparable outcomes in terms of visual and refractive results, long-term stability, total aberrations and optical quality. However, CT LUCIA 611P IOL had significantly less internal coma and SA, unfolding time and uneventful IOL insertions. The Blueject injector appears to meet the expectations of providing a predictable means of IOL delivery.This may be considered a significant advantage, as increased manipulation of foldable IOLs within their injectors may render the IOLs more prone to damage and increase the risk of inadvertent intra-operative complications. Further studies may be beneficial to understand its long-term safety, efficacy, optical quality, PCO behaviour and capsular bag stability, in comparison with other concurrent monofocal IOL technologies.
LIST OF ABBREVIATIONS
| OSI | = Objective Scatter Index |
| MTF | = Modular Transfer Function |
| PCO | = Posterior Capsule Opacification |
AUTHORS' CONTRIBUTIONS
Sheetal Brar – Data analysis, Manuscript writing, Statistical analysis, Review of literature.
Sri Ganesh- Concept and Design, Critical revision of manuscript.
Smith Snehal Sute- Data collection, Statistical analysis, Review of literature.
Swati Chidre- Data collection.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This prospective study was approved by the institutional ethics committee of Nethradhama Eye Hospital, Bangalore.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
All patients provided written informed consent.
STANDARDS OF REPORTING
STROBE guidelines were followed
AVAILABILITY OF DATA AND MATERIALS
Supplementary material is available on the Publisher’s website.
FUNDING
None.
CONFLICT OF INTERESTS
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


