RESEARCH ARTICLE
Surgical Outcome of Combined MicroPulse Transscleral Laser Therapy with Phaco Emulsification in Patients with Cataract and Glaucoma
Ahmed Al Habash1, *, Wael Otaif2
Article Information
Identifiers and Pagination:
Year: 2022Volume: 16
E-location ID: e187436412209260
Publisher ID: e187436412209260
DOI: 10.2174/18743641-v16-e2209260
Article History:
Received Date: 13/5/2022Revision Received Date: 5/7/2022
Acceptance Date: 17/8/2022
Electronic publication date: 21/11/2022
Collection year: 2022

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose:
To determine the effectiveness and safety of combined MicroPulse transscleral laser therapy (TLT) and phacoemulsification in patients with co-existing cataracts and glaucoma.
Methods:
A retrospective consecutive case series of 22 eyes of 19 patients with co-existing cataract and glaucoma. The patients underwent MicroPulse TLT, phacoemulsification, and intraocular lens implantation during the same setting. A comparison of baseline data with the data at 18 months follow-up was made to determine the variation in best-corrected visual acuity (BCVA), intraocular pressure (IOP), and changes in the number of anti-glaucoma drugs.
Results:
Twenty-two eyes of 19 patients (57.9% were female) underwent combined MicroPulse TLT and phacoemulsification. The mean age was 60.5±9.3 years (range: 39.0 to 76.0). Nine eyes (40.9%) had primary open-angle glaucoma, nine eyes (40.9%) had chronic angle-closure glaucoma, and four eyes (18.2%) had pseudoexfoliation glaucoma. The mean baseline IOP was 26.3±4.7, which was significantly reduced to 15.3±2.4 mmHg (43.9%±10.2%) at 18 months (p<0.001). The median number of glaucoma medications was 4 (2 to 5) at baseline and 2 (0 to 4) at 18 months (p=0.002). The mean BCVA was 0.84±0.31 LogMAR (Snellen: 20/138) at baseline and 0.28±0.23 LogMAR (Snellen:20/38) at 18 months (p<0.001). The mean follow-up period was 15.8±3.0 months (range 12 to 18).
Conclusions:
Combined MicroPulse TLT and phacoemulsification was a safe and effective procedure that achieved reduction in both IOP and glaucoma medications for up to 18 months, with no associated vision-threatening complications.
1. INTRODUCTION
Over the last decade, a wide variety of new laser and surgical treatment options have become available to treat glaucoma. As a result, glaucoma specialists have moved away from traditional treatment options, such as trabeculectomy and other glaucoma incisional surgeries, to non-incisional micro-invasive glaucoma surgeries with better safety profiles and incorporate several novel surgical techniques [1, 2].
Some of the non-incisional glaucoma surgeries available are cyclo destructive procedures that aim to reduce aqueous secretion rate, in contrast to conventional procedures that work at the aqueous outflow. The traditional transscleral cyclo photo coagulation (TSCPC), for instance, is based on reducing aqueous humor production through the use of a continuous-wave diode laser. A traditional TSCPC can, however, result in vision-threatening complications such as uveitis, chronic hypotony, loss of vision, and in rare cases, it may result in phthisis bulbi and sympathetic ophthalmia [3].
Unlike the continuous-wave delivery, the micropulse delivery (MicroPulse TLT) has a lower probability of collateral damage through delivering short bursts of energy to the ciliary body with rest periods to reach a biological response in the ciliary body [4]. MicroPulse TLT has been described as an effective and safe method in treating various glaucoma types with minimal vision-threatening complications [5-7]. Moreover, the efficacy and safety of MicroPulse TLT for lowering intraocular pressure (IOP) in the eye with good visual potential has been demonstrated in various long-term studies [5, 8].
Cataract extraction is an effective method to reduce the IOP as a standalone procedure; it was found to substantially reduce IOP and the amount of glaucoma medication needed for glaucomatous eyes. Studies have revealed an IOP reduction of 9% in patients with glaucoma after cataract surgery [9, 10].
Several studies have demonstrated the effectiveness of combining cataract surgery with the cyclo destructive procedure (endoscopic cyclo photo coagulation (ECP)). This combined procedure lowers IOP and the need for glaucoma medication in patients with various types of glaucoma [11-13].
This retrospective study aimed to determine the safety and efficacy of using a combined treatment approach of MicroPulse TLT, phacoemulsification, and implantation of an intraocular lens (IOL) in patients with cataracts and glaucoma. To our best knowledge, results from this combined treatment option have not yet been published.
2. MATERIALS AND METHODS
2.1. Patients and Study Design
This retrospective consecutive case series included 22 eyes from 19 patients who had concomitant cataract and glaucoma and underwent MicroPulse TLT and phacoemulsification at the King Fahad Hospital of the University (KFHU; Khobar, Saudi Arabia) between August 30th, 2018 and January 1st, 2020. All surgical procedures were performed by a single surgeon (A.H.) in accordance with the Declaration of Helsinki, and the Ethics Committee of KFHU approved the study protocol. The review board waived the consent requirement because of the retrospective nature of the study. The patients' demographic data, including their age and gender, were collected. The preoperative parameters included baseline IOP as assessed with a Goldmann applanation tonometer, glaucoma subtype, number of glaucoma medications (combined medications were counted as two), earlier surgical interventions, and best-corrected visual acuity (BCVA).The post operative parameters included: IOP, number of glaucoma medications, BVCA, and surgical complications. The postoperative follow-up was conducted at 1 week and at 1, 3, 6, 12, and 18 months. The customized data collection sheets were used to report the information. BCVA was converted to a logarithm of the minimum angle of resolution (log MAR) for the analysis. Complete success was defined as an IOP lowering of ≥ 30% from the preoperative reading while the patient remained off all glaucoma medications. Also, qualified success was defined as an IOP lowering of ≥ 30% while remaining on glaucoma medication. Failure was defined as an inability to meet the mentioned criteria for success. The visual acuity loss was defined as a loss of more than two lines on the LogMAR scale of the visual acuity chart.
2.2. Surgical Procedure
The procedure was started with MicroPulse TLT in the main operating room under local anesthesia (a 50:50 mixture of 2% lignocaine with 100micrograms/20ml adrenaline and 0.5% bupivacaine) in the form of peribulbar, retrobulbar, or sub tenon block depending on the axial length of the operated eye. An Iridex Cyclo G6® (IRIDEX Laser Systems) was used in its MicroPulse treatment mode with preset standardized parameters to deliver a power of 2100mW for 110 seconds for every 180-degree arc with a 31.3% duty cycle. A 2% lidocaine gel was applied over the 360° of the palpebral conjunctiva prior to the application using the original MicroPulse P3® Probe. The MicroPulse P3 probe was then placed adjacent to the limbal margin and held perpendicular to the surface which positioned the fiber optic 2.8 mm away from the limbal margin. With a steady and firm pressure, the probe was slid continuously back and forth ten times for a total of 110 seconds (about 10s for every sweep in one direction) for the superior 180°, which was then repeated for the inferior 180°. Care was taken to bypass the 3 and 9 o'clock meridians, the areas of the thinned sclera, the filtering bleb, and the tube shunt device. After completing the MicroPulse TLT procedure, the eye was prepped and draped for the cataract extraction using the routine phacoemulsification technique with a posterior chamber IOL, followed by subconjunctival injection of steroids. The postoperative treatment regimen included 1% topical prednisolone acetate (initially every two hours, tapered over eight weeks) and a fourth-generation topical fluoroquinolone (moxifloxacin) 4 times daily for a week.
2.3. Statistical Analysis
Statistical analysis was performed with IBM SPSS for windows (v.22; IBM Corp, Armonk, NY, USA). All figures were made with Microsoft Excel (2019, Microsoft Corp., USA). The normality of the data was assessed by the Shapiro-Wilk test. Baseline and postoperative IOP and BCVA were compared with paired t-tests, while the comparison of reduction in IOP and improvement in BCVA between eyes with and without prior surgery were compared with unpaired t-tests. The baseline and the postoperative number of glaucoma medications were compared with Wilcoxon signed ranks test while the comparison of numbers of glaucoma medications between eyes with and without prior surgery were analyzed with the Mann-Whitney U test. Reduction in IOP and improvement in BCVA were compared among POAG, CACG, and PXG with one-way ANOVA. Changes in the number of glaucoma medications were analyzed with the Kruskal Wallis test. A p-value of less than 0.05 was considered statistically significant. Variation is cited as standard deviation.
3. RESULTS
The mean age of the patients was 60.5±9.3 years (range: 39.0 to 76.0), and 57.9% were female. A total of 22 eyes of 19 patients underwent combined MicroPulse TLT and phacoemulsification. Primary open-angle glaucoma (POAG) and chronic angle-closure glaucoma (CACG) were the most common diagnoses, which were found in 81.8% (n=19) of the eyes. Seven eyes (22.8%) had prior glaucoma surgery, with trabeculectomy (13.6%, n=3), deep sclerectomy (4.6%, n=1), or Ahmed glaucoma valve implantation (4.6%, n=1). The mean follow-up period was 15.8±3.0 months (range: 12 to 18). Preoperative demographics and baseline clinical characteristics are listed in Table 1.
3.1. Treatment Success
Complete success and qualified success rates at different postoperative time points are shown in Fig. (1) and Table 2. One week after the surgery, 86.4% of the operations were deemed a complete success, and 0% were deemed a failure. At 18 months after the operation, 71.4% of the operations were deemed a qualified success and 14.3% a complete success. There was no visual acuity loss in any of the eyes. There were neither retreatments with MicroPulse TLT nor re-operations for glaucoma in any eye that were necessary.
| Variable | - | Data |
|---|---|---|
| Number of eyes (n) | - | 22 |
| Number of patients (n) | - | 19 |
| Age ±SD (year), range | - | 60.5±9.3 (39.0 to 76.0) |
| Gender % (n) | - | - |
| - | Males | 42.1% (8) |
| - | Females | 57.9% (11) |
| Preoperative diagnosis | - | - |
| - | POAG | 40.9% (9) |
| - | CACG | 40.9% (9) |
| - | PXG | 18.2% (4) |
| Cup to disc ratio ±SD, range | - | 0.72±0.15 (0.50 to 0.90) |
| Prior glaucoma surgery | - | - |
| - | Trabeculectomy | 13.6% (3) |
| - | Deep Sclerotomy | 4.6% (1) |
| - | Ahmed valve implantation | 4.6% (1) |
| - | None | 77.3% (17) |
| Follow-up ±SD, range (months) | - | 15.8±3.0 (12 to 18) |
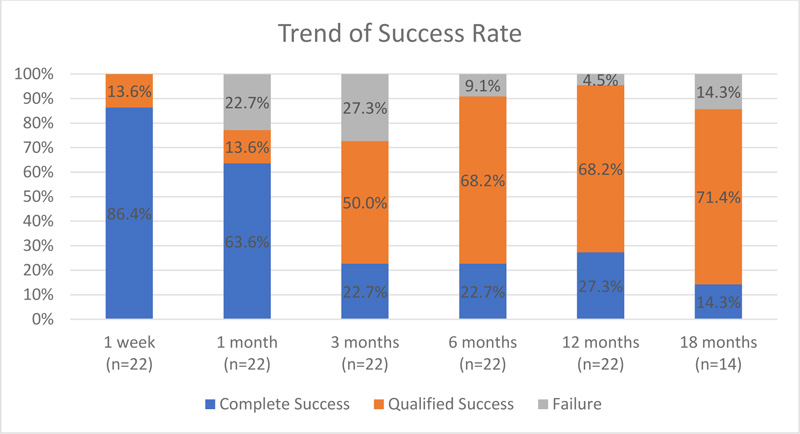 |
Fig. (1). Success rate over 18 months. |
Table 2. Success rate over 18 months.
| Postoperative Time | N | Complete Success | Qualified Success | Failure | ||
|---|---|---|---|---|---|---|
| 1 Week | 22 | 86.4% (19) | 13.6% (3) | 0% (0) | ||
| 1 month | 22 | 63.6% (14) | 13.6% (3) | 22.7% (5) | ||
| 3 months | 22 | 22.7% (5) | 50.0% (11) | 27.3% (6) | ||
| 6 months | 22 | 22.7% (5) | 68.2% (15) | 9.1% (2) | ||
| 12 months | 22 | 27.3% (6) | 68.2% (15) | 4.5% (1) | ||
| 18 months | 14 | 14.3% (2) | 71.4% (10) | 14.3% (2) |
3.2. Intraocular Pressure
There was a significant reduction in IOP after combined phacoemulsification and MicroPulse TLT. The mean IOP at presentation was 26.3±4.7 (range: 20.0 to 37.0). It was reduced significantly to 11.5±2.6mm Hg (a 56.3% reduction) at one week postoperatively, 15.3±3.4 mm Hg (a 41.8% reduction) at 1-month, 15.2±3.4mm Hg (a 42.2% reduction) at the 3-month, 14.5±2.3mm Hg (a 44.9% reduction) at the 6-month, 14.6±2.2mm Hg (a 44.5% reduction) at the 12-month, and 15.3±2.4mm Hg (a 41.8% reduction) at the 18-month follow-up (p<0.001) (Fig. 2 and Table 3). There was no significant difference in IOP reduction between eyes with prior glaucoma surgery (41.9%) or without prior glaucoma surgery (44.7%) with p=0.679. There was also no significant difference in IOP reduction between POAG (34.0%±10.1%), CACG (48.8%±10.4%), and pseudoexfoliation glaucoma (PXG) (45.7%±0.2%) with p=0.077. One eye had transient elevated IOP of 22.0 mmHg three months postoperatively, which returned to 14.0 mmHg at the six months visit with two glaucoma medications.
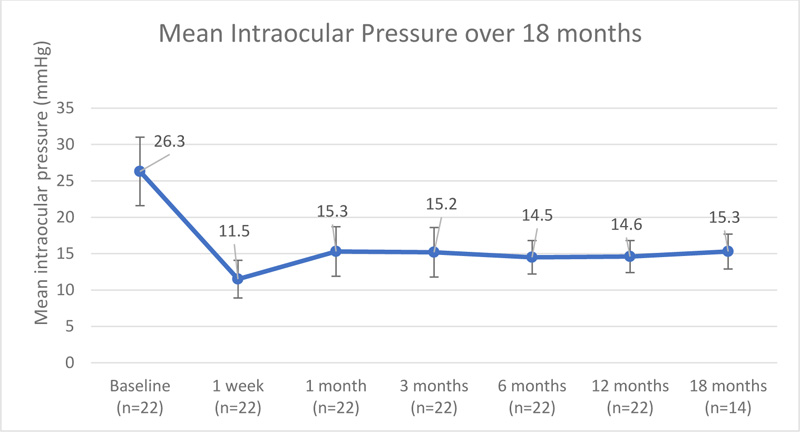 |
Fig. (2). Mean intraocular Pressure over 18 months. |
| Time | Intraocular Pressure (mmHg) | ||||
|---|---|---|---|---|---|
| N | Mean±SD | Median | Minimum | Maximum | |
| Baseline | 22 | 26.3±4.7 | 25.0 | 20.0 | 37.0 |
| 2 weeks | 22 | 11.5±2.6 | 11.0 | 7.0 | 18.0 |
| 1 month | 22 | 15.3±3.4 | 16.0 | 8.0 | 20.0 |
| 3 months | 22 | 15.2±3.4 | 15.5 | 10.0 | 22.0 |
| 6 months | 22 | 14.5±2.3 | 14.5 | 11.0 | 20.0 |
| 12 months | 22 | 14.6±2.2 | 14.5 | 11.0 | 18.0 |
| 18 months | 14 | 15.3±2.4 | 15.5 | 11.0 | 19.0 |
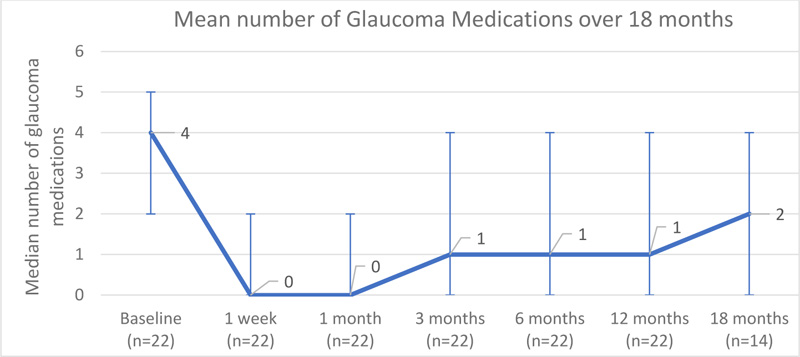 |
Fig. (3). Mean number of glaucoma medication over 18 months. |
| Time | Number of Glaucoma Medications | ||||
|---|---|---|---|---|---|
| N | Mean±SD | Median | Minimum | Maximum | |
| Baseline | 22 | 3.6±0.7 | 4.0 | 2.0 | 5.0 |
| 1 weeks | 22 | 0.2±0.6 | 0.0 | 0.0 | 2.0 |
| 1 month | 22 | 0.2±0.5 | 0.0 | 0.0 | 2.0 |
| 3 months | 22 | 1.1±1.1 | 1.0 | 0.0 | 4.0 |
| 6 months | 22 | 1.4±1.2 | 1.0 | 0.0 | 4.0 |
| 12 months | 22 | 1.5±1.4 | 1.0 | 0.0 | 4.0 |
| 18 months | 14 | 2.1±1.5 | 2.0 | 0.0 | 4.0 |
3.3. Glaucoma medications
There was a significant drop in the number of glaucoma medications used after combined MicroPulse TLT and phacoemulsification; the median number of glaucoma medications preoperatively was 4 (2 to 5), while at 18 months postoperatively, it was 2 (0 to 4) (p=0.002) (Fig. 3 and Table 4). There was no statistically significant difference in the number of glaucoma medications between eyes with prior glaucoma surgery (median: 1.5, range: 0 to 3) and eyes without prior glaucoma surgery (median: 2, range: 0 to 4) with p=0.513. There was also no significant difference in the number of glaucoma medications between POAG (median: 3, range: 2 to 3), CACG (median: 1, range: 0 to 4), and PXG (median: 1, range: 0 to 2) with p=0.138.
3.4. Visual Acuity
There was a significant improvement of 5.7±2.5 lines in BCVA after combined phacoemulsification and MicroPulse TLT; the mean BCVA was 0.84±0.31 LogMAR (Snellen: 20/138) at baseline and 0.28±0.23 LogMAR (Snellen: 20/38) at 18 months (p<0.001) (Fig. 4 and Table 5). Also, there was no statistically significant difference in improvement in BCVA between eyes with prior glaucoma surgery (5.2±1.9 lines) and eyes without prior glaucoma surgery (5.9±2.8 lines) with p=0.649. There was no significant difference in corrected distance visual acuity improvement between POAG (6.3±2.1 lines), CACG (5.4±3.1 lines), and PXG (5.5±2.2 lines) with p=0.870.
| Time | Corrected Distance Visual Acuity in LogMAR (Snellen) | ||||
|---|---|---|---|---|---|
| N | Mean±SD | Median | Minimum | Maximum | |
| Baseline | 22 | 0.84±0.31 (20/138) | 0.70 | 0.20 | 1.30 |
| 1 week | 22 | 0.56±0.24 (20/73) | 0.50 | 0.10 | 1.00 |
| 1 month | 22 | 0.35±0.22 (20/45) | 0.30 | 0.00 | 0.70 |
| 3 months | 22 | 0.29±0.22 (20/39) | 0.25 | 0.00 | 0.70 |
| 6 months | 22 | 0.28±0.22 (20/38) | 0.25 | 0.00 | 0.70 |
| 12 months | 22 | 0.28±0.22 (20/38) | 0.25 | 0.00 | 0.70 |
| 18 months | 14 | 0.28±0.23 (20/38) | 0.25 | 0.00 | 0.70 |
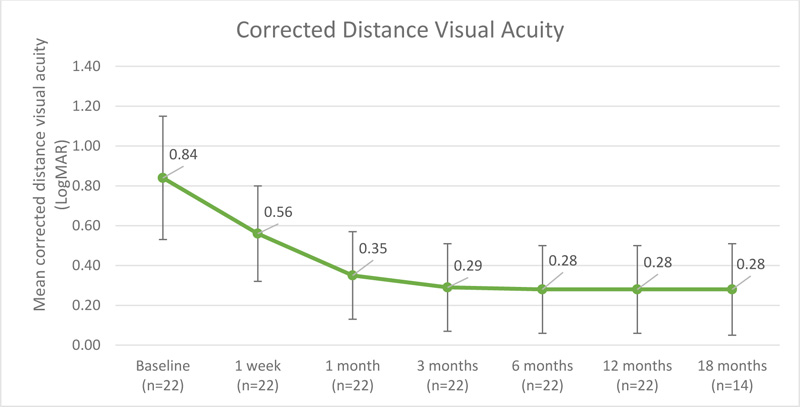 |
Fig. (4). Mean distance corrected visual acuity over 18 months follow-up. |
4. DISCUSSION
Our study constitutes the first retrospective study that involves a single-centre, single-surgeon case series of 22 eyes with concomitant cataracts and glaucoma that were subjected to a combined treatment of MicroPulse TLT and phacoemulsification. This 18-month retrospective series demonstrated a substantial reduction in IOP and glaucoma medications, accompanied by an excellent safety profile.
The mean IOP levels showed a 44.5% and 41.8% reduction at the 12- and 18-month follow-ups. The present study also reported a considerable decline in anti-glaucoma medications; the patients withdrew from an average of two drugs from the baseline at 18 months. This implies that this combined treatment of MicroPulse TLT and phacoemulsification effectively reducing both IOP and the number of glaucoma medications, besides alleviating the burden of glaucoma drugs that affect patient compliance and quality of life. In addition, our results showed a substantial improvement in BCVA with no associated intra-operative complications at the time of cataract surgery, such as zonular dialysis and posterior capsular tear, confirming that combined MicroPulse TLT and phacoemulsification does not interfere with visual expectation after cataract extraction. Moreover, this combined approach ensured that the glaucoma was treated first, followed by cataract extraction. This approach helps in the early glaucoma treatment and reduces surgical time, as initiating the procedure with cataract extraction can delay the glaucoma treatment if associated intra-operative complications develop.
Several long-term studies have demonstrated that MicroPulse TLT is an effective non-invasive glaucoma procedure that achieves a sustained reduction in both IOP and glaucoma medication as the standalone procedure in various subtypes of glaucoma and in eyes with good visual acuity with no vision-threatening complications [5, 8, 14].
We tried to compare our study's outcomes with the outcomes of two earlier studies concerning the early outcome of combined MicroPulse TLT and phacoemulsification that was so far only reported on posters at a conference [15, 16]. The major hurdles for this comparison were that their follow-up periods were shorter than ours and that clinical details had not been published yet. One study reported a 32% IOP reduction at a 3-month follow-up after MicroPulse TLT/phaco [16], while the other study reported a 56% IOP reduction at a 6-months follow-up [15]. Our series demonstrated a 42.2% and 44.9% IOP reduction at 3, and 6 months respectively, which appears to be in a similar range to the previous results.
From the IOP reduction point of view, a useful comparison for MicroPulse TLT/phaco might be with Phaco/ECP and Phaco/MIGS procedures, which are similar to MicroPulse TLT in that they also aim for rapid and safe IOP reduction without affecting the success of later incisional glaucoma surgery. One study described the outcome of a 3-year-follow up of 84 patients who underwent combined phaco/ECP for uncontrolled glaucoma, in which an IOP reduction from 18.7 mmHg at the baseline to 14 mmHg (25% reduction) was reached, with no significant drop in the mean number glaucoma medications [12]. In addition, the Manchester iStent study considered 41 patients that underwent phacoemulsification and iStent implantation with a follow-up period of 3 years. The study outcomes showed that the average IOP at the baseline was 21.2 mm Hg, which was reduced to 17.1 mm Hg (19.3%) at the 3-year follow-up, with the mean number of glaucoma medications reduced from 2.1 at baseline to 1.3 [17]. In the present study, considering MicroPulse TLT/phaco, the mean reduction in IOP levels was found to be 11 mm Hg (43.9%) at 18 months follow-up. Moreover, the mean number of glaucoma medications was reduced from 4 at baseline to 2 at 18 months of follow-up. This implies that MicroPulse TLT/phaco resulted in a more significant reduction in both IOP and glaucoma medications than the Phaco/ECP and Phaco/Istent procedures.
The present series included various subtypes of glaucoma; nine eyes (40.9%) had POAG, nine eyes (40.9%) had CACG, and four eyes had PXG (18.2%). There was, however, no significant difference in the reduction of IOP, glaucoma medications, or BCVA, implying that this combined approach is suitable for the treatment of various glaucoma subtypes.
In our series, five eyes (22.8%) had undergone prior glaucoma surgery, namely trabeculectomy, deep sclerectomy, or Ahmed glaucoma valve implantation. In these eyes, the success rate was similar to eyes without prior glaucoma surgery. MicroPulse TLT/phaco is, therefore a suitable treatment for patients who had previously failed glaucoma surgery, especially considering that repeated incisional glaucoma surgery can result in many complications besides having a reportedly low success rate.
Our study did not report any vision-threatening complications such as hypotony, intraocular inflammation, cystoid macular edema, or loss in visual acuity. This low complication rate may be attributed to our early intensive topical steroid treatment and less intense micropulse treatment protocol.
CONCLUSION
MicroPulse TLT/phaco conducted by a single surgeon with a provided fixed parameter resulted in favorable outcomes. The results of the last follow-up revealed a steady decline in IOP levels as well as a decrease in the number of anti-glaucoma drugs taken. The use of MicroPulse TLT/phaco in our study for treating various types of glaucoma also yielded a satisfactory safety profile. Moreover, the treatment combination did not result in any vision-threatening complications. The current study has several limitations, including the retrospective nature of the study, the rather small sample size, and the relatively short follow-up period. This implies the need for further studies with larger sample sizes and longer follow-ups.
LIST OF ABBREVIATIONS
| TSCPC | = Traditional Transscleral Cyclophotocoagulation |
| MicroPulse TLT | = Micropulse Transscleral Laser Therapy |
| ECP | = Endoscopic Cyclophotocoagulation |
| KFHU | = King Fahad Hospital of the University |
| (BCVA) | = Best-Corrected Visual Acuity |
| (log MAR) | = Logarithm of the Minimum Angle of Resolution |
| (POAG) | = Primary Open-Angle Glaucoma |
| (CACG) | = Chronic Angle-Closure Glaucoma |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee of KFHU approved the study protocol.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
The review board waived the consent requirement because of the retrospective nature of the study.
STANDARDS FOR REPROTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study are available within the manuscript.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Vinod K, Gedde SJ, Feuer WJ, et al. Practice preferences for glaucoma surgery. J Glaucoma 2017; 26(8): 687-93. |
| [2] | Coleman AL, Richter G. Minimally invasive glaucoma surgery: Current status and future prospects. Clin Ophthalmol 2016; 10: 189-206. |
| [3] | Aquino MCD, Barton K, Tan AMWT, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: A randomized exploratory study. Clin Exp Ophthalmol 2015; 43(1): 40-6. |
| [4] | Sarrafpour S, Saleh D, Ayoub S, Radcliffe NM. Micropulse transscleral cyclophotocoagulation. Ophthalmol Glaucoma 2019; 2(3): 167-71. |
| [5] | Al Habash A, AlAhmadi A S. Outcome Of MicroPulse® transscleral photocoagulation in different types of glaucoma. Clin Ophthalmol 2019; 13: 2353-60. |
| [6] | Tekeli O, Köse HC. Outcomes of micropulse transscleral cyclophotocoagulation in primary open-angle glaucoma, pseudoexfoliation glaucoma, and secondary glaucoma. Eur J Ophthalmol 2020; 31(3): 1113-21. |
| [7] | Kaba Q, Somani S, Tam E, Yuen D. The effectiveness and safety of micropulse cyclophotocoagulation in the treatment of ocular hypertension and glaucoma. Ophthalmol Glaucoma 2020; 3(3): 181-9. |
| [8] | Varikuti VNV, Shah P, Rai O, et al. Outcomes of micropulse transscleral cyclophotocoagulation in eyes with good central vision. J Glaucoma 2019; 28(10): 901-5. |
| [9] | Armstrong JJ, Wasiuta T, Kiatos E, Malvankar MM, Hutnik CML. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: A systematic review and meta-analysis of 3-year data. J Glaucoma 2017; 26(6): 511-22. |
| [10] | Azuara BA, Burr J, Ramsay C, et al. Effectiveness of early lens Extraction For The Treatment Of Primary Angle-Closure Glaucoma (EAGLE): A randomised controlled trial. Lancet 2016; 388(10052): 1389-97. |
| [11] | Rathi S, Radcliffe NM. Combined endocyclophotocoagulation and phacoemulsification in the management of moderate glaucoma. Surv Ophthalmol 2017; 62(5): 712-5. |
| [12] | Smith M, Byles D, Lim LA. Phacoemulsification and endocyclophotocoagulation in uncontrolled glaucoma: Three-year results. J Cataract Refract Surg 2018; 44(9): 1097-102. |
| [13] | Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg 2014; 40(8): 1313-21. |
| [14] | Zaarour K, Abdelmassih Y, Arej N, Cherfan G, Tomey KF, Khoueir Z. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma 2019; 28(3): 270-5. |
| [15] | Parkhomenko AKO, Parkhomenko G. Safety and efficacy of combined transscleral micropulse diode laser cyclocoagulation with phacoemulsification and intraocular lens implantation. Official ESCRS | European Society of Cataract & Refractive Surgeons. Available from: https://www.escrs.org/paris2019/programme/poster-village-details.asp?id=32861 (Accessed on: Oct. 27, 2020). |
| [16] | Daas SLA, Nagar A, Ho H, Amon A, Versi I, Gadhvi K. Early results of phacoemulsification combined with micropulse cyclodiode laser in patients with glaucoma: Efficacy and safety Official ESCRS | European Society of Cataract & Refractive Surgeons Available from: https://www.escrs.org/paris2019/programme/poster-village-details.asp?id=33840 (Accessed on: Oct. 27, 2020). |
| [17] | Tan SZ, Au L. Manchester iStent study: 3-year results and cost analysis. Eye 2016; 30(10): 1365-70. |







