RESEARCH ARTICLE
Biometric Measurement of Iridocorneal Angle Parameters in a Sample of Healthy Saudi Eyes
Medhat A. Bakr1, *, Ussama Al Naqeeb2, Abdullah Al Mulla2
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e18743641264550
Publisher ID: e18743641264550
DOI: 10.2174/0118743641264550231120135146
Article History:
Received Date: 21/06/2023Revision Received Date: 11/09/2023
Acceptance Date: 02/10/2023
Electronic publication date: 27/11/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Iridocorneal angle (ICA) or anterior chamber angle is the anatomical structure responsible for aqueous humor outflow and regulating intraocular pressure inside the eye.
Objective:
The study aims to evaluate iridocorneal angle parameters in healthy Saudi eyes using the spectral domain located in the anterior segment optical coherence tomography (AS-OCT) and to describe their correlation with other factors.
Methods:
For precise measurements of ICA, anterior segment imaging was executed with the aid of spectral-domain AS-OCT MS-39®. The measurements of the iridocorneal angle included angle opening distance (AOD) and trabecular-iris space area (TISA), located 500 and 750 μm away from the actual scleral spur from nasal and temporal angles.
Results:
The comparison of temporal and nasal angle parameters showed significantly higher AOD500 values in the temporal compared to the nasal angle (p=0.005). The study also evaluated correlations with a grade of the angle, and no correlations were found between gender, refractive error, iris thickness, and angle parameters. Multiple linear regression with backward elimination of non-significant factors found age (p=0.002), angle grade (p<0.001), predicting factors for nasal AOD500 (Adjusted R2=0.772, p<0.001), and angle grade (p<0.001) and temporal iris thickness (p=0.004) predicting factors for temporal AOD500 (Adjusted R2=0.511, p<0.001). IOP (p =0.026) and angle grade (p<0.001) were found to predict factors for nasal TISA500 (Adjusted R2=0.639, p<0.001), and angle grade (p<0.001) and temporal iris thickness was found predicting factors for temporal TISA500 (Adjusted R2=0.314, p<0.001).
Conclusion:
AS-OCT is a valuable, easy, non-aggressive method to evaluate the iridocorneal angle.
1. INTRODUCTION
Iridocorneal angle (ICA) or anterior chamber angle is the anatomical structure responsible for aqueous humor outflow and regulating intraocular pressure inside the eye [1]. ICA anatomy plays a vital role in the pathogenesis of different types of glaucoma (especially angle closure). In the Far East, ICA closure is considered a crucial feature for primary angle-closure glaucoma, identifying COL18A1 as an ICA candidate gene for angle closure in humans. Therefore, the assessment of ICA is an essential part of the ophthalmic examination [2, 3]. A gonioscopy examination is a gold standard for assessing the angle. However, it is uncomfortable for some patients due to the huge measurement discrepancy between the examiners [4]. With the advancement of new technology, anterior segment optical coherence tomography (AS-OCT) is increasingly used in the ophthalmic field to obtain precise anterior chamber angle measurements [5]. It is a private and secure imaging modality that offers many useful trabecular-iris space area (TISA) and ICA parameters, such as trabecular-iris angle (TIA), iris thickness (IT), iris volume (IV), iris curve (IC), and angle opening distance (AOD) [6].
Studying the normal angle and providing baseline normative data for the general population will help understand and diagnose ICA-related diseases and identify their risk factors. Many existing studies evaluated the ICA by AS-OCT; they showed a discrepancy in their measurements, which is explained by the difference in ethnicity [7-17]. Considering the Saudi population, some ICA parameters, namely (IT) and (IV), were measured in healthy eyes [18, 19]. However, other parameters of ICA (specifically AOD and TISA) need further evaluation in the Saudi population. Thus, this study aims to provide population-based reference data of iridocorneal angle parameters measured in healthy Saudi eyes with spectral-domain AS-OCT MS-39® (Costruzione Strumenti Oftalmici, Florence, Italy).
2. MATERIALS AND METHODS
2.1. Study Design and Targeted Population
This prospective cross-sectional population-based observatory study was conducted at Dhahran Eye Specialist Hospital from (December) 2021__ till (April) 2022__. The ethical approval was taken from the Institutional Review Board (IRB) of the Dhahran Eye Specialist Hospital (DESH), located in Dhahran, Saudi Arabia (Approval No: 1088/2021_______), and the study followed the guidelines of the statements of Helsinki. Eighty-seven healthy participants were included to conduct this study, and the “RaoSoft” online software calculator was used to calculate the sample size, which was 85 at a 5% confidence interval, 5% error margin, and 50% distribution of responses. The study considered healthy volunteers 18 years or older, with no ocular diseases (except mild refractive errors of three dioptres), with at least best-corrected visual acuity (BCVA) of 20/32 eyesight or better on the Snellen scale. No existing anterior segment pathology was found on slit-lamp examination (except mild irritational conditions such as dryness or blepharitis), intraocular pressure (IOP) by Goldmann applanation tonometry (GAT) of ≤ 21 mmHg, no significant media opacity that obscures the view to the fundus, and no existing retinal or optic nerve head pathology was found. Additionally, participants with a clinical history of vision loss due to ocular trauma, ocular surgeries, lasers, and medications that affect pupil size were not included in this study. Two observers observed the study measurement, and Cohen’s Kappa coefficient was 0.76. All participants received a detailed explanation of the nature of the study. Written consent was obtained before participation and consecutively alternating randomized for right and left eyes.
2.2. Data Collection
Participants who met inclusion criteria underwent complete ophthalmic examination, including best-corrected visual acuity (BCVA) evaluated by Snellen chart and transformed to log MAR, intraocular pressure (IOP) measured by Goldmann applanation tonometry (GAT), gonioscopic examination under mesopic condition using non-indentation Goldmann 3 mirror lens, and detailed slit-lamp biomicroscopic inspection of anterior and posterior sections. For precise measurements of ICA, anterior segment imaging was performed with the aid of a spectral domain known as AS-OCT MS-39® (Costruzione Strumenti Oftalmici, Florence, Italy). Fig. (1) shows an example of ICA measurements taken by the machine from one of the study participants. All images were taken under mesopic light conditions with the participant sitting. Multiple images were taken to ensure the highest image quality, with the scleral spur visible as a reference point. After acquiring the image, a manual location of the scan line was obtained to bisect the eye pupil. Also, the manual location of the scleral spur and peak of the iris’s recess was needed to have automatic machine calculation of the angle parameters. Angle opening distance (AOD) and trabecular-iris space area (TISA) were taken from temporal and nasal angles at 500 m and 750 m from the scleral spur location. In addition, nasal and temporal iris thickness (IT) were measured in this study. Subjects were directed to be stable in the primary position by looking at the fixation target (internal light source), then identifying scleral spur SS at both sides in horizontal gaze (0-180 degrees).
Two lines at each side of SS were drawn perpendicularly to the horizontal line connecting SS on both sides. After that, the outline boundary of the anterior and posterior iris surface was drawn. The other two lines parallel the line connecting the lateral anterior iris surface and pupillary border at two levels of the anterior and posterior iris surface. Iris thickness was measured as the vertical line length between the previous two parallel lines at different points (200 points).
2.3. Statistical Analysis
Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 22.0) was used to evaluate the results. (Armonk, NY: IBM Corp.) Continuous variables were summarized with their respective mean and standard deviation alongside categorical variables with percentages. The normality of the data acquired was checked with the Shapiro-Wilk normality test. Paired data that was not normally distributed was compared with the Wilcoxon Signed Ranks test. In addition, the correlation for non-normally distributed data was calculated with Spearman's Rho. To compare categorical variables, a Chi-square test was performed on categorical data. Figures for pictorial representation of the results were created using Microsoft Excel (2019, Microsoft Corp.). A p-value obtained with less than 0.05 significance level was considered statistically significant.
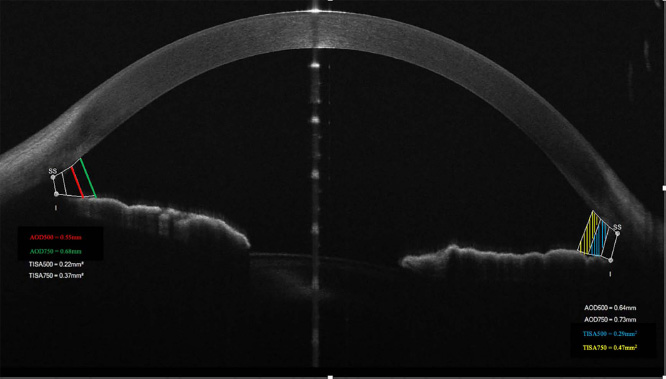 |
Fig. (1). Example of iridocorneal angle (ICA) measurements taken from one of the study participants by MS-39® AS-OCT. |
3. RESULTS
Eighty-seven optical reports from 87 participants were assessed in this study. Demographics and characteristics reported from the clinical trial of study participants are demonstrated in Table 1. There was an insignificant difference in the percentages of males (55.2%) and females (44.8%) included in this study (p=0.335). The mean age of the participants was 41.1 ± 14.6 (18 to 78) years. Patients showed excellent visual acuity (20/20 to 20/32), low cylinder (≤1.00D), and normal intraocular pressure (9.0 to 20.0mmHg). The mean axial length was 24.3 ± 0.33mm. Table 2 compares nasal and temporal angle parameters with their mean values. Our participants had almost insignificant differences between nasal and temporal values except for AOD500, which was higher in the temporal angle (p=0.005). The correlations between patient factors and the angle parameters are demonstrated in Table 3. There were moderate to strong positive correlations (r = 0.6 to 0.9) between the grade of the angle and the nasal and temporal angle parameters indicative of higher angle grade (wider angle) association with more extensive angle parameters. Fair negative correlations (r = -0.4) existed between age and angle parameters (Fig. 2); older age is associated with smaller angle parameters. Also, there were fair negative correlations (r = -0.3 to -0.4) between IOP and angle parameters; higher IOP was associated with smaller angle parameters (Fig. 3).
| Parameter | Level | Statistics | |
|---|---|---|---|
| - | - | % (n) | Mean ± SD (range) |
| Age, year | - | - | 41.1 ± 14.6 (18 to 78) |
| Gender | Males | 55.2 (48) | - |
| Female | 44.8 (39) | - | |
| Eye | Right | 49.4 (43) | - |
| Left | 50.6 (44) | - | |
| BCVA*, Log MAR | - | - | 0.01 ± 0.04 (0 to 0.20) |
| - | - | Snellen:20/21 (20/20 to 20/32) | |
| Equivalent to Spherical, D** | - | - | -0.80 ± 0.77 (-3.25 to +1.00) |
| Cylinder, D** | - | - | -0.42 ± 0.35 (-1.00 to 0) |
| IOP***, mmHg**** | - | - | 14.0 ± 2.53 (9.0 to 20.0) |
| Grade of the angle | - | - | 2.90 ± 0.85 (1 to 4) |
| Iris thickness, µm | Nasal | - | 324 ± 61 (204 to 481) |
| Temporal | - | 342 ± 56 (234 to 520) | |
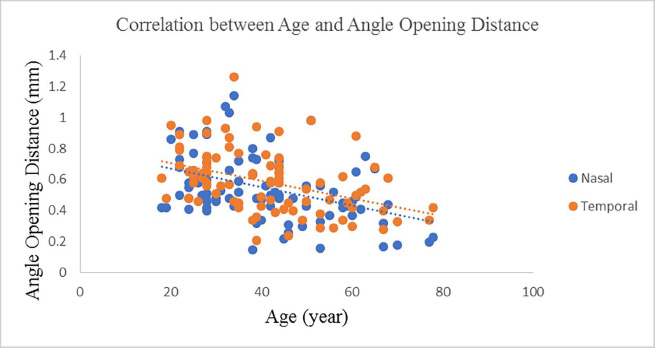 |
Fig. (2). Scatterplot diagram demonstrating the correlation between Age and Angle Opening Distance (AOD). |
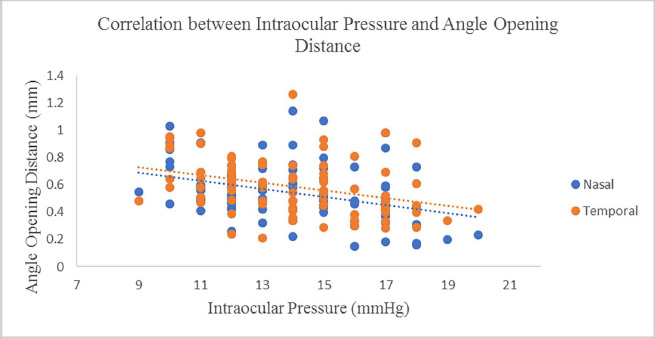 |
Fig. (3). Scatterplot diagram demonstrating the correlation between Intraocular Pressure (IOP) and Angle Opening Distance (AOD). |
| Parameter | Nasal | Temporal | Sig. | ||
|---|---|---|---|---|---|
| - | Mean ± SD | Range | Mean ± SD | Range | - |
| AOD500,* mm** | 0.54 ± 0.21 | 0.15 to 1.14 | 0.59 ± 0.20 | 0.21 to 1.26 | 0.005 |
| AOD750, mm | 0.74 ± 0.24 | 0.20 to 1.49 | 0.75 ± 0.25 | 0.34 to 1.65 | 0.488 |
| TISA500,***mm2**** | 0.23 ± 0.08 | 0.08 to 0.45 | 0.24 ± 0.09 | 0.08 to 0.58 | 0.317 |
| TISA750, mm2 | 0.41 ± 0.14 | 0.13 to 0.85 | 0.42 ± 0.16 | 0.17 to 1.05 | 0.987 |
| Factor | Nasal | Temporal | ||||||
|---|---|---|---|---|---|---|---|---|
| - | AOD500* | AOD750* | TISA500** | TISA750** | AOD500* | AOD750* | TISA500** | TISA750** |
| Age | -0.398 (p<0.001) | -0.396 (p<0.001) | -0.390 (p<0.001) | -0.379 (p<0.001) | -0.457 (p<0.001) | -0.407 (p<0.001) | -0.473 (p<0.001) | -0.463 (p<0.001) |
| Gender | -0.029 (p=0.786) | -0.094 (p=0.385) | -0.055 (p=0.613) | -0.097 (p=0.373) | -0.109 (p=0.314) | -0.149 (p=0.168) | -0.119 (p=0.273) | -0.173 (p=0.110) |
| Eye | 0.029 (p=0.788) | -0.001 (p=0.990) | 0.067 (p=0.540) | 0.031 (p=0.778) | 0.013 (p=0.906) | -0.004 (p=0.970) | -0.017 (p=0.876) | -0.069 (p=0.527) |
| BCVA*** | 0.065 (p=0.549) | -0.063 (p=0.565) | 0.017 (p=0.878) | -0.064 (p=0.558) | 0.035 (p=0.746) | -0.025 (p=0.818) | -0.007 (p=0.948) | -0.057 (p=0.597) |
| IOP**** | -0.368 (p<0.001) | -0.385 (p<0.001) | -0.332 (p=0.002) | -0.359 (p=0.001) | -0.407 (p<0.001) | -0.366 (p<0.001) | -0.411 (p<0.001) | -0.356 (p=0.001) |
| Angle grade | 0.860 (p<0.001) | 0.797 (p<0.001) | 0.817 (p<0.001) | 0.776 (p<0.001) | 0.716 (p<0.001) | 0.694 (p<0.001) | 0.558 (p<0.001) | 0.547 (p<0.001) |
| SE****** | -0.040 (p=0.710) | -0.075 (p=0.488) | -0.060 (p=0.578) | -0.082 (p=0.452) | -0.050 (p=0.644) | -0.082 (p=0.452) | -0.073 (p=0.502) | -0.018 (p=0.870) |
| Cylinder | 0.058 (p=0.592) | 0.096 (p=0.379) | 0.045 (p=0.679) | 0.129 (p=0.234) | 0.094 (p=0.388) | 0.120 (p=0.270) | 0.089 (p=0.411) | 0.109 (p=0.313) |
| IT-Nasal******* | 0.308 (p=0.004) | 0.330 (p=0.002) | 0.189 (p=0.079) | 0.240 (p=0.025) | 0.348 (p=0.001) | 0.370 (p<0.001) | 0.306 (p=0.004) | 0.347 (p=0.001) |
| IT -Temporal******* | 0.161 (p=0.138) | 0.207 (p=0.054) | 0.025 (p=0.817) | 0.109 (p=0.316) | 0.277 (p=0.009) | 0.275 (p=0.010) | 0.272 (p=0.011) | 0.303 (p=0.004) |
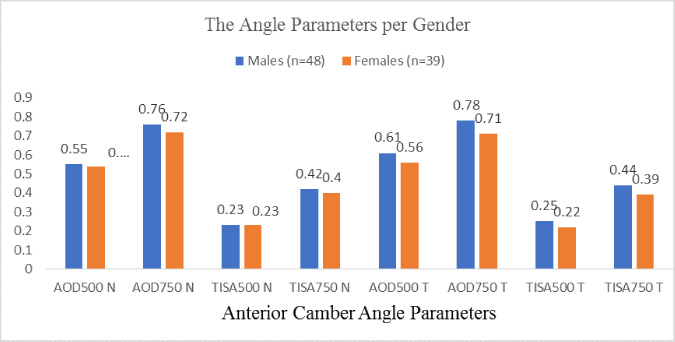 |
Fig. (4). Diagram showing the poor correlation between gender and angle parameters. |
The results also showed no significant differences between genders in angle parameters (Fig. 4), representing poor correlations (r = 0 to 0.2). No correlations were found among laterality, BCVA, spherical equivalent, cylinder, and angle parameters. Multiple linear regression with backward elimination of non-significant factors found age (p=0.002) and angle grade (p<0.001) as predicting factors for nasal AOD500 (Adjusted R2=0.772, p<0.001). Multiple linear regression with backward elimination of non-significant factors found angle grade (p<0.001) and temporal iris thickness (p=0.004) as predicting factors for temporal AOD500 (Adjusted R2=0.511, p<0.001). Multiple linear regression with backward elimination of non-significant factors found IOP (p =0.026) and angle grade (p<0.001) as predicting factors for nasal TISA500 (Adjusted R2=0.639, p<0.001). Multiple linear regression with backward elimination of non-significant factors found angle grade (p<0.001) and temporal iris thickness as predicting factors for temporal TISA500 (Adjusted R2=0.314, p<0.001).
4. DISCUSSION
Measurement of Iridocorneal angle (ICA) appeared to help evaluate and establish the diagnosis of any glaucoma [20]. Recent studies showed that ethnicity is an important discriminating factor to be considered when assessing ICA (Caucasians were found to have wider angles than others) [9-16]. Due to our population's lack of reference normative data for ICA parameters, such a study was necessary. This study analyzed the ICA parameters using AS-OCT on the Saudi population, which is a unique contribution to the literature. AS-OCT usage in angle-closure subjects to examine angle requires comprehensive angle biometry assessment through accurate credentials of the scleral spur as the sclera point of the inward protrusion [21]. In our study, we analyzed the AOD500, AOD750, TISA500, and TISA750 from temporal and nasal angles (from 0 to 180 degrees), which were found to be adequate for assessing the angle by many studies [10, 12, 17]. However, other studies included ICA measurement from superior and inferior quadrants, which might be susceptible to measurement errors due to the need for eyelid manipulation [9, 12]. In our study, the mean angle values were higher compared to what was reported by Leung et al. [17]. However, they were found to be substandard compared to those reported by other studies found in the literature. This could be explained by anatomical variation between different ethnic groups [10-12].
Our study found insignificant differences between temporal and nasal values except for AOD500, which was higher in the temporal angle (p=0.005). This result agreed with Romkens et al. [16], which reported higher values in the temporal angle. However, this contrasts with several other studies reporting lower values in the temporal area [12, 22-24]. Regarding the correlation between age and angle values, our study suggested a strong negative correlation between age and angle values; that is, older age is associated with smaller angle parameters. The lens thickness increases with aging might explain this negative correlation, and several studies strongly agree with this correlation [11, 12, 23]. The study also indicated a strong and positive correlation between the grade of the angle (based on Shaffer classification) and angle values, and a higher angle grade (wider angle) was associated with larger angle values. Friedman et al. [24] reported a strong correlation between gender and angle values; women have narrower angles than men; however, Ruiz-Belda et al. [25] reported a highly significant difference between genders regarding nasal IA measurements at three different meridians. However, our study found a poor correlation between gender and angle parameters, with no significant difference between males and females. A Chinese-American study examined AS-OCT images and found no statistically significant difference in biometric measurements pre and post-gender and age adjustment in detecting primary angle-closure glaucoma [26]. Another study highlighted the significance of angle assessment in the dark to light in attenuating beneficial angle-widening results in PACD eyes to closely evaluate the angle-closure mechanism [27]. Friedman et al. [28] stressed the dangerously increasing rate of Primary angle-closure glaucoma (PACG), a vision loss problem, in older adults across Asia. The study reviews the PACG research and elaborates on improving the imaging of the anterior segment to identify the angle closure and the treatments that are approached to cure PACG [28].
Our study found no correlation between spherical equivalent, cylindrical power, and angle parameters. This contrasts with other studies that established an impact of refractive status on angle measurements (hyperopes found to have shallower angles) [11, 24]. We attribute our finding to the fact that all participants enrolled in our study were chosen to have minimal refractive error, which was not the case in other studies. Lastly, our study had several limitations. The first limitation is that our measurements of angle parameters were limited to nasal and temporal angles, with no measures taken from superior and inferior quadrants. Due to the poor quality images attained for the superior and inferior quadrants and the requirement for eyelid manipulation, all quadrants needed to be evaluated in cases of glaucoma. The second limitation is that iris volume (IV) and curve (IC) were not included in our study, which could dynamically influence the angle. Also, our study used subjective utilization of the angle imaging and manual localization of the scleral spur, which could induce measurement errors. However, this was the limitation of the other studies in the relative literature [9-11, 15, 17].
CONCLUSION
In conclusion, AS-OCT was a valuable, easy, non-invasive method to evaluate the iridocorneal angle. We provided population-based reference data of iridocorneal angle parameters, which can be used in future studies. Our study suggests that age, angle grade, CDR, and IOP influence iridocorneal angle measurements.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical approval was taken from the Institutional Review Board (IRB) of Dhahran Eye Specialist Hospital (DESH), Dhahran, Saudi Arabia (Approval No: 1088/2021).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All procedures performed in studies involving human participants were by the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all participants.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared None.







