All published articles of this journal are available on ScienceDirect.
Clinical Outcomes of Phacoemulsification using Dropless Cataract Surgery Compared to Traditional Protocol using Preoperative and Post-operative Eye Drops
Abstract
Objective:
This study compared the clinical outcomes of phacoemulsification performed with two different protocols, including dropless cataract surgery using intraoperative intraocular injections, versus the traditional protocol using preoperative and post-operative topical corticosteroids and antibiotics.
Methods:
This was a retrospective cohort that was conducted at the Ophthalmology Department of Maghrabi Hospital, Al-Madinah, Saudi Arabia, between date 2015 and date 2020. All consecutive eyes that underwent phacoemulsification cataract surgery using either dropless (Group 1) or traditional (Group 2) protocol were included. Preoperative and early postoperative (<1 month) measurements of visual acuity and intraocular pressure were analyzed as the primary study outcomes.
Results:
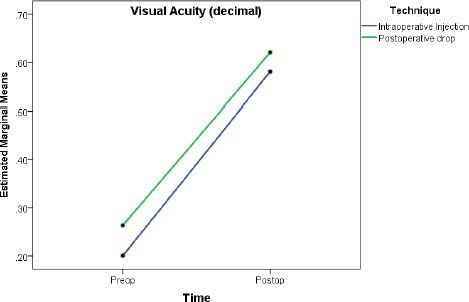
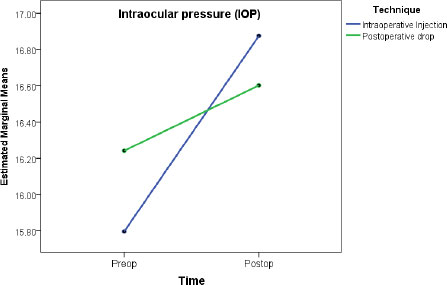
A total of 577 eyes were included: 207 (51.1%) in the dropless and 198 (48.9%) in the traditional protocol groups. Postoperative assessments showed no difference in visual acuity or IOP between the two groups. Pre-to-postoperative analysis showed an increase in mean IOP by 0.72 mmHg (p=0.002) and an increase in median VA by 0.47 decimal (p<0.001), with no difference between the two groups (p>0.05). Similarly, factorial repeated-measure ANOVA analysis showed no effect for the protocol type on IOP change (between-subject analysis, p=0.742), while time had a small effect (partial eta squared =0.018, p=0.002). Regarding visual acuity, the protocol type showed a small effect (between-subject effect: partial eta squared = 0.017, p=0.002), while time had a large effect (partial eta squared = 0.635, p<0.001).
Conclusion:
In summary, our study compared dropless cataract surgery with traditional methods and found no significant differences in postoperative outcomes. Dropless surgery simplifies care, reduces the risk of non-compliance, and is potentially cost-effective. However, it highlighted the need for optimized follow-up schedules. Future research should explore long-term effects, conduct cost-effectiveness analyses, and assess patient satisfaction to comprehensively evaluate the impact of these surgical approaches on both healthcare efficiency and patient well-being.
1. INTRODUCTION
Cataract surgery is among the most common surgeries in developing countries [1]. In 1967, Dr. Charles Kelman, an American ophthalmologist, was the first to introduce phacoemulsification as a novel cataract surgical procedure, which is characterized by the use of an ultrasound-guided needle to emulsify and aspirate the lens via a small corneal incision and implant an intraocular lens [2]. Nonetheless, phacoemul- sification has numerous complications, including corneal edema, postoperative uveitis, intraocular pressure (IOP) elevation, cystoid macular edema, and posterior capsule opacification [3], which may require preventive strategy with topical drugs.
To minimize the risk of such complications, nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and antibiotics are used as adjunctive therapies to cataract surgery. The administration of NSAIDs in cataract surgery showed positive effects on pain, intraoperative miosis, postoperative inflammation, and cystoid macular edema (CME) [4]. The American Academy of Ophthalmology (AAO), via its “Cataract in the Adult Eye Preferred Practice Pattern®” of 2016, supported the use of NSAIDs alone or in combination with topical corticosteroids to reduce the risk of postoperative CME [5]. This was supported by a previous systematic review from the AAO [1] Treatment with topical corticosteroids was demonstrated to decrease post-cataract surgery inflammation [6]. Topical antibiotics are also used by the majority of surgeons for the prophylaxis of postoperative endophthalmitis after cataract surgery, as reported by the American Society of Cataract and Refractive Surgery (ASCRS) in 2014 [7].
However, these therapies are exposed to multiple adverse events [1]. For instance, the classic topical corticosteroids (i.e., dexamethasone, prednisolone, and fluorometholone) are associated with increased IOP after cataract surgery, especially in “steroid responders” who can exhibit high levels of IOP even with small doses or short duration [6]. Such risk is less common with certain newer topical corticosteroids, such as rimexolone and loteprednol etabonate [6]. Regarding NSAIDs, corneal melts or NICM (NSAID-induced corneal melts) have been shown to be one of the most serious specific complications [8]. These adverse effects are to be added to phacoemulsification surgery complications, which include the early postoperative elevation in IOP, typically peaking at 3 to 7 hours after surgery when using topical corticosteroids [9]. While no treatment regimen was found to efficiently prevent IOP flares, particularly in glaucomatous patients [3], the use of corticosteroids may further increase this risk. Moreover, the contribution of eyedrops to the total cataract surgery expenditure is significantly high [10].
Despite the aforementioned observations, insufficient research efforts have been allocated towards investigating the effects of dropless surgeries in reducing the occurrence of postoperative complications, as well as optimizing the cost-effectiveness of ophthalmic surgical interventions. On the other hand, the visual outcomes following cataract surgery vary significantly and seem to be influenced by the use of a topical regimen in combination with the surgical procedure [11]. Moreover, the benefits of giving up corticosteroid eyedrops on postoperative IOP, as well as the other visual outcomes, have not been sufficiently explored. Hence, surgeries with preoperative, perioperative and postoperative drops, and drop-free approaches (dropless cataract surgery) may have different results [11].
Moreover, dropless cataract surgery eliminates the need for patients to administer postoperative drops, thereby reducing the risk of non-compliance. This aspect of dropless cataract surgery not only simplifies the postoperative care process but also addresses the potential challenges associated with patient adherence to the prescribed drop regimen [12]. Therefore, exploring the impact of dropless procedures compared to traditional protocols that involve the use of eye drops is crucial in understanding the benefits and potential advantages of this approach in cataract surgery.
The present study aimed to compare the clinical outcomes of phacoemulsification performed with two different protocols, including dropless cataract surgery, using intraoperative intraocular injections, versus the traditional protocol, using preoperative and post-operative eye drops.
2. METHODS
2.1. Design and Setting
This was a retrospective cohort that was conducted at the Ophthalmology Department of Maghrabi Hospital, Al-Madinah, Saudi Arabia, between date 2015 and date 2020. The study was ethically approved by the institutional review board of Taiba University (Ref #TU-037-22).
2.2. Population
The study population comprised consecutive eyes that underwent phacoemulsification cataract surgery, with either the dropless or traditional protocol and each eye was considered as an individual study subject. To be eligible for inclusion, subjects had to meet the following criteria: undergoing unilateral or bilateral cataract extraction, being willing and able to administer eye drops as instructed and attend scheduled visits, and having the potential for postoperative best-corrected visual acuity of 20/30 or better. Exclusion criteria consisted of the following: children, as they are known to exhibit a heightened steroid response based on existing literature; the presence of severe preoperative ocular pathology, such as amblyopia, proliferative diabetic retinopathy, macular edema, uveitis, glaucoma, pseudoexfoliation syndrome, pigment dispersion syndrome, history of steroid response, optic nerve atrophy, macular degeneration, or epiretinal membrane; use of systemic or topical medications known to interfere with vision; intraocular conventional surgery performed within the past three months or intraocular laser surgery performed within the past one month. All consecutive eligible eyes that underwent surgery throughout the study period were included in the analysis.
2.3. Groups
The eyes included in the study were divided into two groups based on the protocol used, which was determined according to the surgeon's preferred technique:
2.3.1. Dropless Protocol
After the uneventful phacoemulsification procedure, intraocular lens implantation, and removal of the ophthalmic viscosurgical device (OVD), an intracameral injection of triamcinolone acetonide 1.2mg in 0.03 ml was delivered to the anterior chamber.
2.3.2. Traditional Protocol
Eyes in this group followed the traditional protocol. After cataract removal through phacoemulsification and intraocular lens implantation, patients were instructed to use topical prednisolone acetate 1% four times a day for one week. The dosage was gradually tapered down over a four-week period. Additionally, patients were prescribed moxifloxacin eye drops to be used four times a day for one week.
2.4. Data Collection
A structured Excel sheet was designed to collect the following data: patient’s characteristics, including age, gender, comorbidities; treated eye characteristics, including laterality (unilateral or bilateral), side; and preoperative visual acuity (in decimal scale) and IOP; protocol including dropless (group 1) or traditional (group 2); and postoperative outcomes including visual acuity (in decimal scale), IOP (mmHg), and follow up time in days.
2.5. Statistical Methods
Data were analyzed with the Statistical Package for Social Sciences version 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to present categorical data as frequencies and percentages and continuous data as mean (standard deviation [SD]) or median (interquartile range [IQR]) depending on the normality of the distribution. The two groups, dropless versus traditional, were compared for both baseline and outcome variables; the chi-square test was used for categorical variables, the independent t-test was used for normally distributed continuous variables, and the Mann-Whitney test was used for non-normally distributed ones. Baseline-to-postoperative changes in IOP and visual acuity were analyzed using paired t-test and Related-Samples Wilcoxon Signed Rank test, respectively. The effect of the protocol on the pre-to-postoperative change in IOP and AV was analyzed using Factorial Repeated-Measure ANOVA, with the calculation of the effect size using Pillai’s Trace statistics. A p-value of <0.05 was considered to reject the null hypothesis.
3. RESULTS
3.1. Participants’ Characteristics
A total of 577 eyes were operated involving 405 patients, with a mean (SD) age of 66.23 (10.03) years, with no significant difference between the two groups (p=0.767). Gender distribution showed female predominance (56.5%) with no significant difference between the two groups (p=0.993). Patients from the postoperative drops group had a higher prevalence of comorbidities (56.6%) compared with those of the intraoperative injection group (56.5%), and the difference was statistically significant (p=0.043). The most common comorbidities included hypertension (37.5%) and diabetes mellitus (33.8%). However, there was no difference in the number of comorbidities between the two groups (p=0.148).
With respect to operated eyes, surgery was unilateral in 57.5% of the cases, involving the right (51.5%) and left (48.5%) eyes equally. The distribution by intervention type was equal, showing 207 (51.1%) versus 198 (48.9%) in intraoperative injection versus postoperative drops, respectively (Table 1).
| - | - | Total | Intraoperative Injection | Postoperative Drops | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Parameter | Level | Mean | SD | Mean | SD | Mean | SD | |
| Age | (years), range = 25-96 | 66.23 | 10.03 | 66.38 | 10.61 | 66.08 | 9.41 | .767 |
| Parameter | Level | N | % | N | % | N | % | p-value |
| Gender | Male | 176 | 43.5 | 90 | 43.5 | 86 | 43.4 | - |
| - | Female | 229 | 56.5 | 117 | 56.5 | 112 | 56.6 | .993 |
| Comorbidities | None | 174 | 43.0 | 99 | 47.8 | 75 | 37.9 | - |
| - | Yes | 231 | 57.0 | 108 | 52.2 | 123 | 62.1 | .043* |
| - | HTN | 152 | 37.5 | 72 | 34.8 | 80 | 40.4 | .243 |
| - | DM | 137 | 33.8 | 67 | 32.4 | 70 | 35.4 | .525 |
| - | Heart disease | 30 | 7.4 | 11 | 5.3 | 19 | 9.6 | .100 |
| - | Other | 29 | 7.2 | 12 | 5.8 | 17 | 8.6 | .277 |
| No. Comorbidities | 0 | 174 | 43.0 | 99 | 47.8 | 75 | 37.9 | - |
| 1 | 133 | 32.8 | 61 | 29.5 | 72 | 36.4 | - | |
| 2 | 79 | 19.5 | 40 | 19.3 | 39 | 19.7 | - | |
| 3+ | 19 | 4.7 | 7 | 3.4 | 12 | 6.1 | .148 | |
| Treated eyes | Unilateral | 233 | 57.5 | 124 | 59.9 | 109 | 55.1 | - |
| - | Bilateral | 172 | 42.5 | 83 | 40.1 | 89 | 44.9 | .323 |
| Eye side§ | OD | 297 | 51.5 | - | - | - | - | - |
| - | OS | 280 | 48.5 | - | - | - | - | - |
| Technique | Intraoperative INJ | 207 | 51.1 | - | - | - | - | - |
| - | Postoperative drops | 198 | 48.9 | - | - | - | - | - |
| Parameter | Level | Total | Dropless (N=290) | Traditional (N=287) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| - | - | Medial | IQR | Medial | IQR | Medial | IQR | - |
| Visual acuity | (Decimal) | 0.20 | 0.24 | 0.13 | 0.24 | 0.20 | 0.35 | .014*M |
| - | - | Mean | SD | Mean | SD | Mean | SD | - |
| IOP | (mmHg) | 15.99 | 3.21 | 15.75 | 2.81 | 16.22 | 3.53 | .088 t |
| - | - | N | % | N | % | N | % | - |
| Eye side | OD | 297 | 51.5 | 180 | 51.7 | 147 | 51.2 | - |
| - | OS | 280 | 48.5 | 140 | 48.3 | 140 | 48.8 | .903 |
| IOP level | Normal (10-22) | 548 | 98.4 | 273 | 99.6 | 275 | 97.2 | - |
| - | Moderate (22-26) | 5 | 0.9 | 1 | 0.4 | 4 | 1.4 | - |
| - | High (>26) | 4 | 0.7 | 0 | 0.0 | 4 | 1.4 | .059 |
* Statistically significant difference.
3.2. Preoperative Ophthalmological Parameters
The median visual acuity (in decimal) was significantly (p=0.014) higher in traditional (0.20 [IQR=0.35) compared with the dropless group (0.13 [0.24]); however, no statistically significant difference was observed in mean IOP (p=0.088) or the percentage of eyes with moderate or high IOP (p=0.059) (Table 2).
3.3. Early Postoperative Ophthalmological Parameters
Postoperative assessments showed no difference in visual acuity or IOP between the two groups. However, postoperative follow-up was significantly delayed in the eyes from the dropless group, as the majority were followed up during week 2 postop (59.9%) or later (12.2%); on the other hand, the majority of eyes in the traditional group were followed up at day 1 (23.4%) or week 1 (64.4%), and this difference was statistically significant (p<0.001) (Table 3).
3.4. Baseline to Early Postoperative Change in AV and IOP
Regardless of the protocol, both IOP and VA increased from baseline to postop; the mean IOP increased by 0.72 mmHg (p=0.002), and the median VA increased by 0.47 decimal (p<0.001). This resulted in an increased percentage of high IOP, from 0.7% to 2.6% (p<0.001) (Table 4).
| Parameter | Level | Total | Dropless (N=290) | Traditional (N=287) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |||
| Visual acuity | (Decimal) | 0.67 | 0.40 | 0.67 | 0.34 | 0.67 | 0.40 | .051 M |
| - | - | Mean | SD | Mean | SD | Mean | SD | - |
| IOP | (mmHg) | - | - | 16.86 | 3.92 | 16.64 | 5.47 | .578 t |
| - | - | N | % | N | % | N | % | - |
| IOP level | Normal (10-22) | 510 | 92.9 | 260 | 93.2 | 250 | 92.6 | - |
| - | Moderate (22-26) | 25 | 4.6 | 13 | 4.7 | 12 | 4.4 | - |
| - | High (>26) | 14 | 2.6 | 6 | 2.2 | 8 | 3.0 | .829 |
| FU time | Day 1 | 122 | 21.6 | 57 | 19.9 | 65 | 23.4 | - |
| - | Week 1 | 202 | 35.8 | 23 | 8.0 | 179 | 64.4 | - |
| - | Week 2 | 186 | 32.9 | 172 | 59.9 | 14 | 5.0 | - |
| - | Week 3 or later | 55 | 9.7 | 35 | 12.2 | 20 | 7.2 | <.001* |
* Statistically significant difference.
IOP: Intraocular pressure; VA: visual acuity.
| Parameter | Preoperative | Postoperative | - | p-value | ||
|---|---|---|---|---|---|---|
| - | Mean | SD | Mean | SD | Mean change | - |
| IOP | 16.02 | 3.22 | 16.74 | 4.73 | +0.72 | .002* pt |
| - | Median | IQR | Median | IQR | Statistics | - |
| VA (decimal) | 0.20 | 0.24 | 0.67 | 0.40 | 18.87 | <.001* W |
| - | N | % | N | % | Statistics | - |
| IOP level | - | - | - | - | - | - |
| Normal (10-22) | 548 | 98.4 | 510 | 92.9 | - | - |
| Moderate (22-26) | 5 | 0.9 | 25 | 4.6 | - | - |
| High (>26) | 4 | 0.7 | 14 | 2.6 | 3.569 | <.001*W |
* Statistically significant difference.
IOP: Intraocular pressure; VA: visual acuity.
| - | Dropless (N=290) | Traditional (N=287) | - | ||
|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | p-value |
| VA (decimal) | 0.38 | 0.29 | 0.36 | 0.28 | .325t |
| IOP | 1.08 | 4.45 | 0.36 | 6.18 | .124t |
| Parameter | Median | IQR | Median | IQR | p-value |
| VA (decimal) | 0.38 | 0.42 | 0.34 | 0.50 | .179 W |
| IOP | 1.00 | 5.00 | 0.00 | 6.00 | .026* W |
IOP: Intraocular pressure; VA: visual acuity.
* Statistically significant difference.
3.5. The Protocol Effect on Surgical Outcomes
With respect to the protocol, the pre-to-post-operative change in VA (p=0.179) and IOP (p=0.124) was comparable in the two protocols. However, the median IOP change was significantly higher in the dropless group (median change in IOP = 1 versus 0) compared with the traditional technique group respectively (p=0.026, Related-Samples Wilcoxon Signed Rank test) (Table 5).
In Factorial repeated-measure ANOVA analysis, the protocol showed no effect on IOP change (between-subject analysis, p=0.742), while time had a small effect (partial eta squared =0.018, p=0.002) (Table 6, Fig. 1). Regarding VA, the protocol showed a small effect (between-subject effect: partial eta squared = 0.017, p=0.002), while time had a large effect (partial eta squared = 0.635, p<0.001) (Table 6, Fig. 2).
| Analysis/Outcome | Factor |
Statistics (Pillai’s Trace)§ |
p-value | Partial Eta Squared | Comment (Effect Size) |
|---|---|---|---|---|---|
| IOP | Time | 0.018 | .002* | 0.018 | Small effect |
| Technique (between-subjects) | - | .742 | 0.000 | No effect | |
| Time x technique | 0.004 | .124 | 0.004 | No effect | |
| Visual Acuity (decimal) | Time | 0.635 | <.001* | 0.635 | Large effect |
| Technique (between-subjects) | - | .002* | 0.017 | Small effect | |
| Time x technique | 0.002 | .325 | 0.018 | No effect |
§ Test of equality of covariance matrices showed sig.>0.001 (assumption violated); thence, Pillai’s Trace was used to estimate the effect size.
* Statistically significant result.
4. DISCUSSION
4.1. Summary of the Findings
One of the benefits of the dropless cataract surgery approach is to eliminate the need for patients to administer postoperative drops, resulting in a reduction in the risk of non-compliance and subsequent poor outcomes. To complete the comparative approach, our study compared the postoperative visual outcomes between steroid and antibiotic drops combined with phacoemulsification and drop-free phacoemulsification in cataract surgery. Irrespective of the protocol, early postoperative assessments showed a significant improvement in VA associated with an increase in IOP. However, the dropless group – that received corticosteroids and antibiotics intraoperatively – was likely to induce a greater increase in the median IOP without showing a significant effect in Repeated-Measure ANOVA analysis. Moreover, the postoperative follow-up of the dropless group was significantly delayed (2 weeks or longer versus 1 day to 1 week) compared with the traditional protocol group, which is explained by a difference in routine follow-up practice between the two techniques.
4.2. Adverse Events of Anti-inflammatory Eyedrops in the Setting of Cataract Surgery
The most reported adverse events linked with corticosteroid eye drops in the context of cataract surgery are an increase in IOP, subconjunctival hemorrhage, iritis, and the occurrence of white deposits around the eye (18). Furthermore, topical corticosteroids can have systemic effects when administered with high frequency or duration [13]. A 75-year-old woman developed a maniac episode 9 days after taking prednisolone 1% eye drops following cataract surgery [14]. Jinagal et al. reported a clinical case of a male infant aged 6 weeks who received topical steroids after undergoing phacoaspiration with primary posterior capsulotomy for congenital bilateral cataracts, followed by bilateral membranectomy two and four weeks later. After 6 to 8 weeks of receiving topical steroids, the patient exhibited symptoms of intraocular hypertension and experienced an increase in body weight with a cushingoid appearance [13].
Topical NSAIDs have also been linked with numerous side effects, including conjunctival stinging, burning, hyperemia, contact dermatitis (less frequently), and corneal complications, such as corneal anesthesia, superficial punctate keratitis, corneal infiltrates, and epithelial defects [15]. One of the most serious adverse effects that can lead to blindness is NICM, which has been reported by several studies [15]. NICM is described in two stages: the epithelial stage, in which corneal epithelial defect, reduced eicosanoid levels, leukocyte infiltration, and matrix metalloproteinases are observed, and the stromal stage, marked by matrix metalloproteinases mediated degradation of stromal collagen [15]. Recently, it has been demonstrated that topical NSAIDs can delay corneal wound healing and promote corneal injury healing [16]. Ashena et al. reported a case of an 84-year-old diabetic female who developed severe corneal melt and perforation on the fifth postoperative day after receiving topical NSAIDs prophylaxis for routine cataract surgery in her second eye, which progressed to vision loss due to recurrent corneal melt and chronic choroidal effusions [8].
4.3. Mechanism of Steroid-induced Rise of IOP in Postoperative Cataract Surgery
Corticosteroid drops are used pre-, intra- and or post-operatively during cataract surgery due to their strong potential to decrease ocular inflammation caused by surgical trauma [6]. However, they can increase IOP, possibly by causing stasis in the outflow of aqueous humor through the deposition of extracellular materials [6]. This effect is suggested to result from the downregulation of phagocytosis and metalloproteinase-mediated degradation by the Schlemm's canal endothelial cells (also called trabecular meshwork endothelial cells), along with disrupted homeostasis of the juxtacanalicular tissue, leading to increased aqueous humor flow resistance at the Schlemm's canal’s level [6, 17, 18]. At the molecular level, steroids act on their intracellular receptors in the Schlemm's canal cells, causing an increase in nuclear size and DNA content, which impacts the ability of these cells to proliferate, migrate and phagocytize, thus compromising their regulation function of the aqueous outflow [6, 18]. Furthermore, steroids increase the expression of myocilin gene (MYOC) that encodes for myocilin or trabecular meshwork-inducible glucocorticoid response (TIGR) protein [18]; when dysfunctional, this protein is involved in glaucoma pathogenesis, causing apoptosis and matrix dysfunction to the trabecular meshwork, which results in intraocular pressure dysregulation [19]. In summary, steroids can cause raised IOP by lowering trabecular meshwork cellularity and accumulation of extracellular debris, disrupting the normal flow of aqueous humor, which increases fluid passage resistance.
4.4. Steroid Responders
It is estimated that 5% of the population can develop steroid-induced ocular hypertension and are commonly known as ‘steroid responders’, a complication that occurs more often with the topical than the systemic route [18]. Steroid responders have a particularly higher risk of postoperative acute elevation of IOP, probably because of a higher response of the previously exposed pathophysiological mechanisms in the trabecular meshwork endothelium and juxtacanalicular tissue, leading to greater resistance to the aqueous outflow [20]. As there is no available tool to screen for these patients, they are usually diagnosed after developing a disproportionate rise in IOP for moderate corticosteroid therapy [6, 20]. A recent study found that corticosteroid response occurred among 8.4% of glaucomatous patients after uneventful cataract surgery versus only 2.1% of non-glaucomatous patients [21]. Another study, including 1118 participants, showed that 3.2% developed an adverse response to topical prednisolone eye drops, where the mean IOP jumped from 14.67 to 21.33 mmHg between the pre- and postoperative times [22]. Other patient-related factors, such as age and the presence of other ophthalmological conditions, may contribute to raising this risk. Older individuals are at greater risk of developing reactions compared to younger ones [18]. This corresponds to the category of patients usually receiving cataract surgery. In a retrospective study involving 1642 patients who underwent cataract surgery, 2% (39/1642) were diagnosed as steroid responders. The same study showed that patients aged < 65 years with severe myopia had a 39-fold risk of developing postoperative IOP >28 mmHg and a 35-fold risk of an IOP >35 mmHg compared to non-myopic older patients [23].
4.5. Evidence of Dropless Surgery Benefits in Cataract Patients
Findings from the present study were not conclusive regarding the effect of dropless surgery in reducing postoperative IOP. Except for a greater increase in the median IOP in the dropless surgery, compared to the traditional protocol, which was paradoxical, no significant result was observed in the repeated measure analysis. These findings should account for the significantly delayed follow-up time in the dropless protocol, which would probably bias the outcome comparison between the two groups. By contrast, the literature shows dropless eye surgery to be likely associated with a decrease in postoperative IOP, as compared to the use of eye drops. A prospective randomized controlled trial by Jesper et al. compared the results of phacoemulsification using dropless surgery with 4 age-matched groups of patients who benefited from the following topical regimens: steroid + NSAID in preoperative; steroid + NSAID in postoperative, NSAID alone in preoperative, and NSAID along in postoperative. Although the dropless group had less inflammation control, the groups that received a steroid (preoperatively or postoperatively) experienced higher IOP, while there was no difference in postoperative visual acuity improvement. Another important finding is that preoperative eyedrops did not have effects on early postoperative anterior chamber inflammation [24]. Salimi et al. compared the visual outcomes of trabecular micro-bypass stents and concomitant cataract surgery in patients who received postoperative steroids versus the nonsteroid group and observed more frequent IOP spikes among the steroids group at 1 week postoperatively, with similar visual outcomes between the two groups [25]. Another prospective, non-controlled trial including 200 eyes showed good efficacy and safety of dropless cataract surgery, with normal IOP at all visits and 96% of the patients achieving an uncorrected visual acuity (UCVA) greater than 6/9 3 months after surgery [20]. Another study suggested that a single subconjunctival injection was equally effective in preventing inflammation compared with prolonged topical steroid use [26].
4.6. Dropless Surgery as a Cost-saving Strategy
The cost-effectiveness of corticosteroid drops as adjunctive therapy to cataract surgery should be revised. These drugs are prescribed mainly to prevent postoperative clinically significant eye inflammations; however, such complications have an overall low incidence and are self-limited in most of the cases [19]. A cross-sectional analysis among Medicare beneficiaries showed that the overall cost of postoperative cataract surgery eye drops in one year was more than $167 million, with topical antibiotics being the most prescribed (89%), followed by topical corticosteroids (86%) and NSAIDs (66%). However, corticosteroids (37%) and NSAIDs (36%) accounted for the greatest shares of the expenses compared to antibiotics (26%) [10]. The Ophthalmic Instrument Cleaning and Sterilization (OICS) Task Force, in its 2020 statement, recommended the reduction of the unnecessary wastes of topical drugs in ophthalmology surgery, shedding light on their high economic and environmental burden [27]. According to data obtained from the practice pattern studies among members of the ASCRS (American Society of Cataract Refractive Surgery), Canadian Ophthalmological Society and ESCRS (European Society of Cataract Refractive Surgery), the use of postoperative drugs varies among different countries and surgeons with no standard protocol [28]. This exposes the inappropriate, unnecessary long-term prescribing of eye drops, specifically corticosteroids, expanding the costs and the risk of complications of these drugs. From such a perspective, dropless surgery would be an interesting strategy to reduce topical drugs' economic burden.
5. LIMITATIONS
The present study has two major limitations. The first limitation is the failure to explore and analyze the other potential factors and confounders of IOP; this limitation is due to the retrospective design. The second limitation is the difference in follow-up time between the two groups, resulting in more delayed postoperative assessment in dropless groups. This is justified by the difference in postop follow-up practices between the two techniques.
CONCLUSION
In summary, our study compared dropless cataract surgery with traditional methods and found no significant differences in postoperative outcomes. Dropless surgery simplifies care, reduces the risk of non-compliance, and is potentially cost-effective. However, it highlighted the need for optimized follow-up schedules. Future research should explore long-term effects, conduct cost-effectiveness analyses, and assess patient satisfaction to comprehensively evaluate the impact of these surgical approaches on both healthcare efficiency and patient well-being.
LIST OF ABBREVIATIONS
| NSAIDs | = Nonsteroidal Anti-inflammatory Drugs |
| IOP | = Intraocular Pressure |
| AAO | = Academy of Ophthalmology |
| CME | = Cystoid Macular Edema |
| ASCRS | = American Society of Cataract and Refractive Surgery |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was ethically approved by the institutional review board of Taiba University (Ref # TU-037-22).
HUMAN AND ANIMAL GUIDELINES
The present study was conducted in accordance with the guidelines of the Helsinki Declaration. No animals were used as subjects for this research.
CONSENT FOR PUBLICATION
Since the study was a retrospective chart review, no specific consent was required for publication as it involved only anonymized data and did not directly involve human subjects.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIAL
The data generated from this study can be made accessible upon reasonable request directed to the corresponding author [S.H].
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


