All published articles of this journal are available on ScienceDirect.
Protein Z Plasma Levels are Not Elevated in Patients with Non-Arteritic Anterior Ischemic Optic Neuropathy
Abstract
Background: Protein Z is a glycoprotein that acts as a co-factor for the inhibition of activated coagulation factor X. Protein Z circulating in abnormal levels has been associated with increased risk for acute ischemic events. Non-arteritic Anterior Ischemic Optic Neuropathy (N-AION) is caused by acute ischemic infarction of the optic nerve head, supplied by the posterior ciliary arteries.
Objectives: The aim was to investigate whether there is an association between N-AION and plasma protein Z levels.
Patients and Methods: Twenty-six cases of confirmed N-AION and fifty-two controls were included in the study group. Protein Z was estimated in thawed citrate plasma on both N-AION cases and controls by an enzyme immunoassay. The imprecision of the estimation was satisfactory (CV = 4, 6%).
Results: The controls’ protein Z values distributed within a range 340 to 4200 ng/ml (median = 1420, mean = 1673, SD = 1040 ng/ml). Patients’ protein Z values distributed within a range 420 to 3600 ng/ml (median = 1030, mean = 1520, SD = 939 ng/ml). There was no statistical difference between the two distributions (Independent t-test, p=0.529).
Conclusion: In our study, protein Z levels are not implicated in the pathogenesis of non-arteritic anterior ischemic optic neuropathy (N-AION).
INTRODUCTION
Protein Z (PZ) is a single chain, vitamin K- dependent glycoprotein that was purified from human plasma in 1984 [1, 2].
The amino acid sequence of PZ shows a wide homology with many vitamin K-dependent coagulation factors, such as factors VII, IX, X and anticoagulant protein C, but the protein does not act as expected for a zymogen form of a coagulation factor. PZ does not serve a proteolytic action because it lacks the proper amino acids residues of a serine protease on the active center [3, 4]. A cofactor role, in the inhibition of activated coagulation factor X (f Xa), dependent on phospholipids and calcium ions, was recognized for PZ in 1998 [5].The inhibitory function is exerted by the Protein Z- dependent protease inhibitor (ZPI), which circulates in the human plasma in a complex with PZ [6].The cofactor action of PZ is manifested after its binding to phospholipids and presumably involves the proper localization of ZPI-PZ complex on the phospholipid surface, for interaction with the fXa [5, 7].
In experimental animal models [8]and in humans [9] several studies have suggested that low plasma PZ levels might contribute to venous or arterial thrombosis.
In cases of venous thrombosis a potential association with low PZ levels was only demonstrated in subgroups of patients (men and individuals older than 55 years) but no effect of a concomitant factor V Leiden (FVL) genotype was detected [10, 11].
A number of clinical studies which explore the role of PZ on coronary heart disease [12], ischemic stroke [11] and deep vein thrombosis [13] patients have been published. Particularly in ischemic stroke, patients or at least subgroups of patients [11] with low levels [14, 15]or high levels [16-18] of PZ have been associated with increased risk of stroke. On the other hand, there are studies that claim no association between PZ levels and the risk for stroke [19].
Although an absolute comparison of these studies is impossible, since a lot of differences exist between them, the opposing results that are presented are scientifically intriguing.
Anterior ischemic optic neuropathy (AION) is caused by acute ischemic infarction of the optic nerve head, which is supplied by the posterior ciliary arteries [20, 21]. There are two different types of AION. The most frequent Non-arteritic anterior ischemic optic neuroparhy (N-AION) and arteritic anterior ischemic optic neuropathy (A-AION) usually associated with giant cell arteritis.
N-AION is the most common acute optic neuropathy in older age groups and may result in severe visual acuity or visual field loss [22].It is characterized by sudden, usually painless visual loss, relative afferent pupillary defect and optic disk edema [23]. Optic nerve atrophy-generalized or sectorial- ensues within the next few weeks.
Arteriosclerosis, hypertension [24] diabetes mellitus, ischemic heart disease, hypercholesterolemia [20], “crowded” optic disk [25], nocturnal hypotension [26] and sleep apnea syndrome [23]have been implicated as risk factors.
To our knowledge, there are extremely limited data, on the role of protein Z in ophthalmic disease. The aim of this study was to investigate whether alterations in protein Z might contribute to the enhanced risk of Non-arteritic anterior ischemic optic neuropathy.
MATERIALS AND METHODOLOGY
Study Design: Diagnostic and Exclusion Criteria
Diagnostic criteria for N-AION patients were as follows: sudden loss of vision, relative afferent pupillary defect, and visual field defects consistent with ischemic optic neuropathy and pale swollen optic disk. To exclude giant cell arteritis, a detailed history concerning the systematic symptoms of giant cell arteritis was aqcuired and C- reactive protein (CRP) levels and erythrocyte sedimentation rate (ESR) were measured. ESR over 35mm/h and CRP levels above 5 mg/L were considered suspicious for A-AION and those patients were excluded. In addition, biopsy of temporal arteries, if necessary, wasperformed. The same criteria along with raised white blood cells (WBC) from the blood count, served to exclude inflammatory conditions in both patients and controls. Other exclusion criteria were liver disease, myeloproliferative disorders and use of anticoagulants and contraceptives. In all patients MRI was performed to exclude demyelinization and intracranial tumors. All the patients are under systematic follow–up in regular intervals in our Eye Clinic.
Laboratory Investigation
Blood samples from patients and controls were collected, approximately 2-3 days after original diagnosis, in sodium citrate and EDTA acid anticoagulants. A sample of citrated plasma from each patient was deep frozen for the determination of PZ.
A complete blood cell count, ESR and CRP were performed in all N-AION patients in a regular basis, using routine laboratory techniques [27].
Search for the thrombophilic polymorphisms for factor V Leiden polymorphism (G1691A) and for prothrombin gene polymorphism G20210A, were done by polymerase chain reaction amplification and restriction enzyme digestion.
Detection of factor V Leiden mutation:
In brief, a 267-basepair (bp) segment of the factor V gene was amplified using specific primers (5'-TGC CCA GTG CTT AAC AAG ACC A-3' and 5'-TGT TAT CAC ACT GGT GCT AA-3'), as previously described [28]. The PCR product (10 mL) was digested with 4 U of DNA restriction enzyme Mnl I (New England Biolabs, Beverly, MA, USA), at 37°C for 16 h, subjected to 2% low melting point agarose (Sigma, MO, USA) gel electrophoresis and viewed under ultraviolet light after staining the gel with ethidium bromide. The digested amplicon from wild-type DNA gives 3 bands of 163, 67 and 37 bp, respectively; in contrast, heterozygous and homozygous FVL mutations show four (200, 163, 67 and 37 bp) and two (200 and 67 bp) bands, respectively.
Detection of prothrombin gene mutation:
For detection of G20210 prothrombin gene mutation, we used a PCR-RFLP method that has been described previously [29]. In brief, a 345-bp genomic DNA fragment encompassing a part of the prothrombin gene that contains the mutation was amplified by PCR using specific primers (5'-TCT AGA AAC AGT TGC CTG GC-3' and 5'-ATA GCA CTG GGA GCA TTG AAG C-3'). The PCR product (10 mL) was digested with 20 U of Hind III (New England Biolabs, Beverly, MA, USA), at 37°C for 16 h, subjected to 3% low melting point agarose gel electrophoresis and read using ultraviolet light. Using this method, the wild-type DNA yields a solitary 345-bp band, heterozygous G20210A mutation yields two bands of 345 and 322 bp, respectively and homozygous mutation only one band of 322 bp.
The plasma PZ concentration was determined by a commercial enzyme immunoassay (Asserachrom Protein Z, Diagnostica Stago, France). This assay utilizes the quantitative sandwich enzyme immunoassay technique. Briefly, the standards and samples were incubated in duplicate wells of microtiter plate precoated with a monoclonal antibody specific for protein Z. Following incubation, repeated washings were performed to remove unbound materials and a second monoclonal antibody coupled with peroxidase directed against another epitope of protein Z was added to the assay plates. This step was followed by additional washes and an equal amount of stabilized chromogen; ortho-phenylenediamine (OPD) and urea peroxide substrate were added to each well. This initiates the development of color, which is halted at a set time by the addition of an acid solution. Optical densities were determined using a programmable micro plate reader (Bio-Tek Instruments, Winooski, Vermont, USA). Protein Z concentrations in the samples were determined by interpolation from individual standard curves. Since the sample size of our study is small, to minimize the inter-assay variation, we determined the protein Z values in one experiment, the same day by the same batch of reagents. One sample of thawedcitrate plasma was examined in duplicate, for each patient or control.Protein Z values were calculated from a standard curve provided by the kit and expressed in ng/ml of plasma. The inter-assay variation calculated from the values of normal plasma was satisfactory (CV= 4.6 %) We considered protein Z deficiency levels < 1000ngr/ml [14].
Statistical Analysis was performed with Statistical Package for Social Sciences (SPSS version 14.0) using parametric (t-test) and non-parametric (Fisher’s exact test) statistic procedures where necessary.P-values less than 0.05 were considered statistically significant.
RESULTS
According to diagnostic criteria, three patients with possible giant-cell arteritis were not included in the study. One more patient with meningioma was also excluded from the study.
Twenty-six patients with definite diagnosis of N-AION and in accordance with the criteria described were included in the study.Those were eighteen males and eight females with a mean age of 65 years (range 47 to 83).
The control group consisted of fifty-two healthy individuals from the same geographic area who were referred to the University Eye Clinic for cataract operation and had no history of N-AION, retinal artery or vein occlusion. The control group consisted of thirty males and twenty-two females with a mean age of 70 years (range 47 to 85).
All patients and controls were free from autoimmune diseases, they were not taking anticoagulants and they had no other clinical or laboratory indication that they were under an acute inflammatory phase. In both groups WBC, ESR and CRP values were in the normal range.
Risk factors for atherosclerosis such as hyperlipidemia, (The United States NCEP guidelines for the diagnosis and treatment of hypercholesterolemia) diabetes mellitus, (Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus) hypertension, (2003 new European Society of Hypertension (ESH)/European Society of Cardiology (ESC) guidelines for the management of arterial hypertension ), defined according to strict criteria, were present in both groups with nearly the same incidence (Table1).
Incidence of Vascular Risk Factors
| Risk Factors | N-AION (n =26) | Controls (n =52) | P-Value |
|---|---|---|---|
| Diabetes melitus | 5(19%) | 11(21%) | 0,84 |
| Hyperlipidemia | 9(35%) | 11(21%) | 0,20 |
| Hypertension | 9(35%) | 16(31%) | 0,73 |
| Ischemic heart disease | 4(15%) | 9(17%) | 0,83 |
The concentration values of plasma PZ for the control group were distributed in a range from 340 to 4200 ng/ml with a mean of 1673 ng/ml (median 1420 ng/ml) and a SD of 1040 ng/ml.
In N-AION patients the values of plasma PZ were also distributed in a range from 420 to 3600 ng/ml with a mean of 1520 ng/ml (median 1030 ng/ml) and a SD 931 ng/ml (Fig. 1). There was not any statistically significant difference between the PZ values of N-AION patients and the controls (Independent t-test, p=0.529).
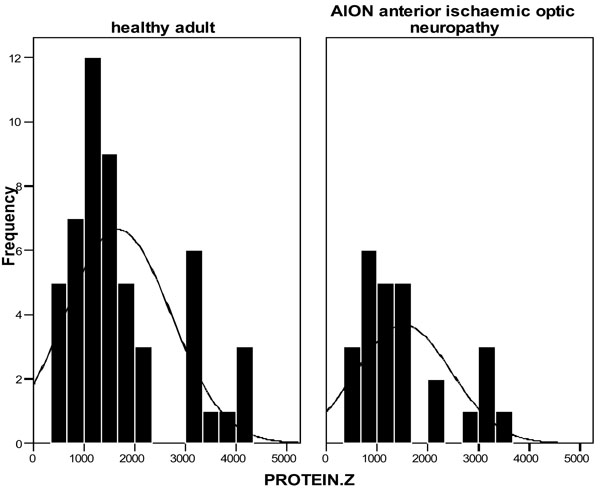
Protein Z levels in controls and N-AION patients.
There was not any statistical difference in PZ levels between males and females in each individual subgroup of N-AION patients and controls (P=0.37 and P=0.55 respectively).
There was no statistically significant difference (P=0.18 using the Kruscal-Wallis test) between the protein Z levels in groups of N-AION patients that had the attack in the left eye, in the right eye or in both eyes.
There was not any statistical difference using the Fisher’s exact test concerning factor V Leiden polymorphism (G1691A) (p=0.26) and prothrombin gene polymorphism G20210A (p=1) between patients and controls.
In two patients and one control subject that were carriers of factor V Leiden polymorphism, the values of protein Z were 1020, 1040 and 960 ng/ml, respectively.
Finally, all patients and controls were negative for lupus anticoagulant.
DISCUSSION
N-AION is the most common acute optic neuropathy in older age groups which can affect both eyes in up to 40% of the patients [30].
Recurrent attacks in the same eye, although uncommon, may also occur [31, 32]. The subsequent visual acuity or visual field loss may be devastating and can lead to serious visual impairment. More than half of the patients discover visual loss upon first awakening or when they first use vision, critically in the morning [23, 26].This was also the case in our study, where 17 (65%) of the patients realized the loss immediately after awakening. The prognosis of N-AION is poor due to optic nerve atrophy ensuing within the next 2-4 weeks, and only a small number of the patients can show significant improvement.
The question whether there is an association between N-AION and thrombophilic risk factors- inherited or acquired- provides quite controversial results. Salomon et al. found no association between N-AION and thrombophilic risk factors [20]. On the other hand, Nagy et al. [21]suggested that hyperfibrinogenemia and factor V Leiden may contribute to the pathogenesis of N-AION, while Van Cott et al. [33] recommend that testing for homocysteine and prothrombin G20210A mutation should be considered in these patients. J.F. Acheson and M.D. Sanders describe seven cases in which there is an associated thrombophilic state and non-arteritic anterior ischemic optic neuropathy [34].
N-AION is a multifactorial disease in which cerebrovascular risk factors such as diabetes mellitus, hypertension, hypercholesterolemia and ischemic heart disease may play an important role. However, because vascular risk factors are more common in individuals of similar age as patients with N-AION, the design of studies of this kind is very difficult to address the issue of whether these systemic factors represent true risk factors for the pathogenesis of N-AION, as Jakobson et al. refer to in their study [35].
This is the first study, to our knowledge, to investigate whether there is an association between N-AION and plasma protein Z levels. The only study concerning PZ in ophthalmic disease, and specificallycentral retinal vein or artery occlusion, was held by Koren-Michowitz et al. [36].
Although protein Z was characterized already in the 1980s, its role in normal and pathologic coagulation is still controversial. There are studies suggesting an association between low plasma PZ levels and acute coronary syndromes [12, 13],pregnancy complications (late fetal demise and intrauterine growth restriction) [37, 38], and ischemic colitis [39].
Opposing results and conclusions, concerning the association of ischemic stroke and PZ plasma concentrations have been reported. The behavior of PZ during the acute phase is also a matter of disagreement [13, 18].
PZ concentration has a broad range in healthy individuals when samples of EDTA or citrate plasma are examined with immunofluoresence [2] or immunoenzymatic techniques [10], respectively. A lot of opposing results also exist, concerning the PZ values on patients with the same disease. Since the inter-assay variation reported for citrate plasma and immunoenzymatic techniques determination is near to 11% [15, 40], a part of this observed variability may be attributed to the unavoidable imprecision of the assays. We were able to minimize this inter-assay variation since we performed only one assay under very low variation (CV=4.6%).
On the other hand the accuracy of determination seems questionable since an International standard for PZ is lacking and as a consequence some researchers report their PZ results as percentages (%) of the mean, probably for comparison reasons [10, 11].
Our results showed no statistically significant difference concerning PZ levels between the N-AION patients and controls. In consistency with this observation are the findings of Lopaciuk et al. 2002 [19] that the values of protein Z are not associated with the risk for stroke in young survivors and the observations of Refaai et al. 2006 [11]who did not find any association of PZ values and risk for stroke or coronary heart disease in older patients.
Contrary to our conclusions, due to small number of patients that we examined, there is always the possibility that some N-AION patients would have lower concentration values of PZ than the controls at the time of the attack. This would be observed in a minority of future patients, probably in less than 5%, according to our results. A none statistically significant trend for an increase of the levels of PZ as the time passed after the eye attack, that we observed, may support this, otherwise, small possibility. In contrast to this hypothesis and in analogy with our results, Refaai et al. 2006 [11] found that PZ levels were not associated with the time of development of coronary heart disease or stroke.
CONCLUSION
In conclusion, we compared a group of carefully selected N-AION patients, without inflammation, with a control group and we did not found a statistically significant difference (p=0.529) between the levels of protein Z.


