All published articles of this journal are available on ScienceDirect.
Long-Term Survivors with Metastatic Uveal Melanoma
Abstract
Background:
To report the tumor, patient, and treatment characteristics of long-term metastatic uveal melanoma survivors.
Methods:
A non-comparative, retrospective case series of patients from a single institution surviving >24 months with metastatic uveal melanoma (UM).
Results:
Nine patients met the study criteria and their charts were reviewed. The mean age at diagnosis of UM was 44.1 years (SD +/- 14.4 years). Initial treatment modalities included enucleation (67%), brachytherapy (22%), and proton beam radiation (11%). The average time from primary tumor diagnosis to detection of metastasis was 125.9 months (SD +/- 95 months). The most common location for initial metastasis was the liver. All patients underwent treatment for metastatic disease including systemic therapy, surgical resection, and isolated hepatic perfusion. The majority of patients received treatment with a tyrosine kinase inhibitor (sorafenib, sunitinib, and/or imatinib). The median survival with metastasis was 51 months (range 27-123 months). Patients had a long disease-free interval before presentation of metastatic disease.
Conclusions:
A small subset of patients with metastatic UM has prolonged survival. Identification of these patients may be helpful for future clinical trial design.
INTRODUCTION
Melanoma of the uveal tract is the most common primary intraocular tumor in adults [1]. Despite advances in treatment of the primary tumor [2-4], there are currently no successful treatment options for metastatic UM. In the Collaborative Ocular Melanoma Study, metastatic spread of the primary tumor occurred in 25% of patients at 5 years and 34% of patients by 10 years [5]. The most common site of initial metastatic spread is the liver, followed by lung and soft tissue sites [6].
Prognosis for metastatic uveal melanoma is poor with 1-year overall mortality of 80 - 87% [5, 7], increasing to 92% at 2 years [5]. A more recent study has reported lower estimated 1-year mortality, closer to 50%, and identified a subset of long-surviving patients with metastatic UM [8]. Factors significantly associated with long survival in that series included patient age younger than 60, increased time to presentation of metastatic disease, initial presentation in lung or soft tissue, treatment with surgery or intrahepatic therapy, and female gender [8].
Very few studies have evaluated the characteristics of long survivors with uveal melanoma. Herein we report on the tumor characteristics, patient factors and treatment regimens of long survivors with UM at our institution.
MATERIALS AND METHODOLOGY
A retrospective chart review was performed of patients from The Ohio State University Medical Center who had a diagnosis of metastasis from uveal melanoma and a survival time of greater than 24 months since the detection of metastatic spread. Institutional review board approval was obtained and the study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Nine patients fit the criteria of long survivors. Records were reviewed for the following information: age at diagnosis, sex, primary tumor height, primary tumor diameter, primary tumor location, primary tumor cell type, treatment modality of primary tumor, time to metastasis (disease-free interval), symptoms at diagnosis of metastatic spread, serum lactate dehydrogenase levels (LDH) levels and serum liver function tests (Aspartate Transaminase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (AP)), previous radiographic studies, location(s) of initial and secondary metastatic spread, treatment regimen(s) of metastatic disease, response to metastatic treatment, and length of survival with metastasis.
RESULTS
Patient Characteristics
The mean age at diagnosis of primary uveal melanoma was 44.1 years (range 26-71 years, SD +/- 14.4 years). The mean age at diagnosis of metastatic disease was 54.6 years (range 29-79.4 years, SD +/- 16.4 years). Five/9 (55%) of the patients were male and four (45%) were female.
Primary Tumor Data
Primary tumor information was available for 8/9 patients. Primary tumor data on patient MM007 was not available as the primary tumor treatment occurred 23 years prior to metastasis and was managed at another facility. The average primary tumor height was 6.3 mm (range 2.0-13.7 mm) and the average maximal basal diameter was 12.4 mm (range 6-18 mm) (Table 1). Initial tumor stage by AJCC classification is listed in Table 1. Seven/9 patients (77%) had choroidal melanoma and one patient (11%) had iris/ciliary body melanoma. One patient developed a second primary uveal melanoma that was restricted to the choroid. Primary tumor cell type data were available in five patients and included mixed (3/5) and epithelioid (2/5). In two additional patients, the cell type of the metastasis was determined to be spindle cell. Three/5 patients had optic nerve infiltration and 2/5 had extrascleral extension.
Patient and Primary Tumor Characteristics
| Patient | UM4033 | MM0001 | MM0002 | UM5061 | UM5043 | MM0008 | MM0007 | MM0004 | MM0005 |
|---|---|---|---|---|---|---|---|---|---|
| Age Diagnosis | 27 | 44 | 44 | 70 | 32 | 64 | 35 | 46 | 35 |
| Sex | Male | Female | Male | Male | Female | Male | Female | Female | Female |
| Tumor Height (mm) | 7.0 | 3.1 | 2.12.8 [1] | 2.0 | 7.0 | 13.7 | Unknown | 11.4 | Unknown |
| Tumor Diameter (mm) | 18.0 | 9.0 | 10.510.0 [1] | 6.0 | 15.6 | 18.0 | Unknown | Unknown | Unknown |
| AJCC Stage | 3 | 1 | 1, 1 [1] | 1 | 3 | 4 | Unknown | Unknown | Unknown |
| Tumor Location | Choroid | Choroid | Choroid | Iris/Ciliary Body | Choroid | Choroid | Unknown | Choroid | Choroid |
| Cell Type | Mixed | Spindle [2] | Unknown | Mixed | Epithelioid | Epithelioid | Unknown | Mixed | Spindle [2] |
| Other Pathologic Features | None | None | None | Extrascleral Extension | Optic Nerve Infiltration | Optic Nerve Infiltration | None | Extrascleral Extension;Optic Nerve Infiltration | None |
| Primary Tumor Treatment | Enucleation | TTT; Brachytherapy | TTT;Brachytherapy | TTT;Enucleation | Enucleation | Enucleation | Enucleation | Enucleation;Orbital Radiation | Proton Beam Radiation |
UM – Pathology specimen from primary uveal melanoma; MM – Pathology from metastatic tumor; AJCC – American Joint Committee on Cancer; TTT- Transpupillary thermal therapy; 1- Second primary choroidal melanoma; 2 – Histopathology from metastatic lesion.
Metastasis Characteristics in Long-Surviving Patients with Metastatic Uveal Melanoma
| Patient | UM4033 | MM0001 | MM0002 | UM5061 | UM5043 | MM0008 | MM0007 | MM0004 | MM0005 |
|---|---|---|---|---|---|---|---|---|---|
| Time to Metastasis (Months) | 25 | 177 | 94 | 56 | 17 | 185 | 273 | 66 | 240 |
| Survival with Mets (Months) | 40 | 97 (a) | 51 (a) | 71 | 27 | 30 | 123 | 49 | 52(a) |
| Initial Metastasis Location | Liver | Liver | Liver | Liver | Liver | Lung | Lung; Breast | Liver | Lung |
| Additional Met Locations | None | Skin; Peritoneum; Lung | None | Bone | Lung | None | Liver; Bone | Bone, adrenal | None |
| Initial # Hepatic Mets | 3 | 3 | 1 | 1 | >5 | N/A | N/A | 3 | N/A |
| Systemic Chemo | TMZ | Taxol;Sorafenib+ Bevacizumab; Sunitinib; | None | TMZ; C/T;DTIC + Sorafenib; Sorafenib;CVD | TMZ; CVD | Imatinib | Sunitinib | C/T +/Sorafenib; Sorafenib | Abraxane;ANA773 Tosylate;Sunitinib |
| Other Treatment | IHP | IHP/GM-CSF; SR; IL-2, PD-1 | SR | SR, IL-2 | IL-2 | StR | SR, TGF- b | SR | SR |
| Initial Response to Treatment | Stable Disease | Disease Regression | No Recurrence | Disease Regression | Disease Progression | Stable Disease | Disease Regression | Stable Disease | No Recurrence |
| LDH | 144 | 155 | 231 (high)* | N/A | 132 | 156 | N/A | 201 (high) | N/A |
| LFTs | AST 21; | AST 18; | AST 34; | AST 24; | AST 21; | N/A | AST 24; | AST 27; | N/A |
| ALT 24 | ALT 18; | ALT 33; | ALT 18; | ALT 26; | N/A | ALT 28; | ALT 34; | N/A | |
| AP 69 | AP 44 | AP 90 | AP 72 | AP 46 | N/A | AP 87 | AP 98 | N/A |
Liver function tests (LFTs) from time of first metastasis presentation: AST – Aspartate Transaminase (ref range: 5-34), ALT – Alanine Transaminase (8-35), AP – Alkaline Phosphatase (38-126); LDH- lactate dehydrogenase (100-190U/L); Other Treatments: IHP – Isolated hepatic perfusion, SC – Systemic Chemotherapy, SR – Surgical Resection, StR – Stereotactic radiotherapy; Biotherapies: Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF), Interleukin 2 (IL-2), Programmed death 1 (PD-1) monoclonal antibody vaccine, Transforming Growth Factor β (TGF-β), and ANA773 Tosylate (Toll-Like Receptor-7 Agonist); Systemic chemo regimens: Temozolomide (TMZ), Paclitaxel (Taxol), Carboplatin + Paclitaxel (C/T), Cisplatin + Vinblastine + Dacarbazine (CVD), Dacarbazine (DTIC), Albumin-Bound Paclitaxel (Abraxane).
* LDH level was in normal range (178) one month later with no intervention.
Six/9 patients (67%) underwent enucleation as treatment for the primary tumor. Of those six patients, one initially had transpupillary thermal therapy (TTT). One patient received pre-enucleation orbital radiation for extrascleral extension. Two/9 patients (22%) underwent multiple modalities of therapy including TTT and brachytherapy with an iodine-125 plaque. One/9 patients (11%) had proton beam radiation.
Metastatic Data
The majority of long-survivors with metastatic UM (8/9, 89%) were asymptomatic at the time of detection of metastatic spread and had metastasis found on routine screening examinations. One patient had pneumonia at the time of detection of pulmonary metastasis.
Initial metastatic spread was noted to the liver in six patients (67%) and the lung in three patients (33%). Metastasis was confirmed either through fine needle aspiration in two patients with liver lesions and through local resection in all others. One patient also had initial metastasis to the breast as well as the lung (Table 2). Five/9 patients (55%) eventually developed metastasis in multiple locations, including liver, lung, bone, skin, and peritoneum. Of patients with initial metastasis to the liver, 5/6 patients had ≤ 3 lesions and one patient had ≥6 lesions (Table 2).
The average time from diagnosis of primary tumor to detection of metastasis was 125.9 months (range 17-273 months, SD +/- 95 months). Four out of the nine patients had >10 years and two had ≥ 20 years between diagnosis of the primary tumor and detection of metastatic disease (Table 2).
Screening bloodwork (e.g., liver function tests (LFTs) and lactate dehydrogenase (LDH)) are relied upon by some clinicians to detect metastatic disease in uveal melanoma patients. Screening bloodwork was evaluated at the time of diagnosis of metastatic disease (Table 2). LDH data were available for 6/9 patients at the time of metastasis diagnosis. The average LDH at diagnosis was 170 U/L (range: 132-231 U/L, SD +/- 38 U/L), in the normal reference range (100-190 U/L). Interestingly, the patient with the highest LDH (231 U/L) underwent repeat testing one month later with a normal result (178), despite having a metastatic liver lesion measuring 1.8 x 1.5cm on CT scan (individual MM0002, Table 2). Other liver function tests were available for 7/9 patients and were found to be within normal limits in all patients (Table 2).
All nine patients underwent treatment for metastatic disease (Table 2). Initial treatment modalities for metastatic disease included systemic chemotherapy or biotherapy in 8/9 (89%), surgical resection in 6/9 (67%), and isolated hepatic perfusion in 2/9 (22%). Of the eight patients who received systemic chemotherapy/biotherapy, 5 also had surgical debulking. The agents utilized for systemic chemotherapy varied (Table 2). Most patients receiving systemic chemotherapy/biotherapy (6/8, 75%) received a tyrosine kinase inhibitor (sorafenib, sunitinib, and/or imatinib) per an approved protocol. Other biotherapy treatments included IL-2 (2/8 patients), IL-2 plus PD-1 monoclonal antibody vaccine (1/8), GM-CSF with isolated hepatic perfusion (1/8), and TGF-β (1/8).
Most patients had a favorable initial response. Initial response to therapy was reported as regression (or no recurrence for initial surgical resections) in 5/9 patients (56%), stable disease in 3 patients (33%), and disease progression in one patient (11%).
The median survival time from the diagnosis of metastasis was 51 months (range 27-123 months, SD +/- 31.8 months). At the time of manuscript submission, four patients are still living.
DISCUSSION
Metastatic UM is a systemic disease that carries with it a very poor prognosis. Herein we report an institutional series of long survivors (>2 years) with metastatic uveal melanoma with an average 51 month survival.
A significant limitation of our study is the small number of patients meeting the survival criteria. This small sample size is related to the poor prognosis of this relatively rare disease. Another limitation is the use of various modalities in the treatment of these patients.
We investigated the primary tumor characteristics in long-survivors with metastatic uveal melanoma. Numerous primary tumor risk factors have been identified that predispose to a poor outcome. Some of these include largest tumor dimension [9], epithelioid cell type [9], ciliary body involvement [9], postlaminar optic nerve invasion [10], extraocular tumor extension [11], older age [12], and male sex [12]. Despite the long survival in our group, the primary tumors had a range of stages and markers for metastatic -disease. Of the six patients that an AJCC primary tumor stage could be determined, three patients had stage 1, two had stage 3, and one had stage 4 disease. There were also two patients with extrascleral extension of the tumor (Fig. 1) and three with optic nerve infiltration. Despite these poor prognostic indicators in the primary tumor, long-term survival was still achieved.
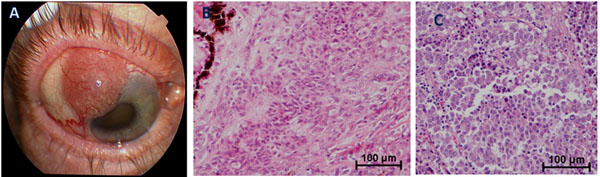
(A) External photograph depicting extrascleral extension of amelanotic iris/ciliary body melanoma; (B) Histologic features of primary tumor showing a mixed cell morphology with anaplastic epithelioid and spindle malignant melanoma cells invading the ciliary body. Choroidal and iris involvement were also present (not shown); (C) Histologic features of metastatic liver lesion showing malignant melanoma cells of epithelioid type with inflammatory infiltrate.
Interestingly, the majority of the primary tumors (7/8) of long survivors originated in the choroid and did not involve the ciliary body. The one tumor in the series that involved the ciliary body may have originated in the iris, but ciliary body origin could not be excluded. Typically UM of ciliary body origin carries significant risk of systemic spread [13]. These tumors tend to have cytogenetic abnormalities that predispose to metastatic disease [14]. Somatic chromosomal abnormalities including monosomy of chromosome 3 and chromosome 8 gains have been found to be predictive of metastatic development [14]. Gene expression profiling has also been a powerful tool allowing clinicians to stratify uveal melanoma patients into two risk groups – high (class 2 tumors) and low risk (class 1 tumors) for metastatic spread [15]. Importantly, monosomy of chromosome 3 in metastatic UM has now been associated with a poor response to metastatic treatment, whereas disomy and partial change of chromosome 3 is associated with a better response [16]. It is plausible that tumors of more posterior choroidal origin may have cytogenetic differences associated with slower growth and longer survival with metastasis.
While initial metastasis to the liver may portend a poorer prognosis than to the lung or soft tissue [8,17], recent research has focused on the number of liver metastases as not only a prognostic factor but a way to guide treatment. Of the 6 patients in this study with initial spread to the liver, 5 had 3 or fewer metastases detected on initial presentation. Numerous studies have showed that increased bulk of liver disease is correlated with survival. Frenkel et al. found that patients with <6 metastatic hepatic lesions have a statistically significant survival benefit over those with 6 or greater hepatic lesions [18]. Aoyama et al. also found prolonged survival in selected patients who underwent metastatectomy of liver lesions for uveal melanoma [19]. Currently, no randomized controlled trial has completely defined the role of surgical resection in patients with metastatic uveal melanoma.
Screening labs, LDH and LFTs were evaluated in our patients. The initial LDH blood work was elevated in 2/6 of patients at the time of detection of metastatic disease and LFTs (AST, ALT, and AP) were within normal limits in all patients with available data. These data support the findings of others that current bloodwork screening tools are not sufficiently sensitive for early detection of metastatic disease [20].
The primary non-surgical means of treatment in this series was systemic chemotherapy (78%) and included a variety of different agents (Table 2). Despite the seeming benefit of this aggressive treatment, a large meta-analysis of literature through 2007 has shown little benefit from therapy [21]. Notably, this study did not evaluate data from some recent therapies which appear more promising such as sunitinib [22].
Despite the poor prognosis of this disease, numerous factors have been associated with prolonged survival of patients with metastatic uveal melanoma. Rietschel et al. found that prolonged survival correlated with lung/soft tissue as first site of metastasis, treatment with surgery or intrahepatic therapy, female sex, age younger than 60, and a longer interval from diagnosis to development of metastatic spread [8]. Our patient group had a number of characteristics predisposing to prolonged survival that agreed with previous studies including younger patient age (mean 44.1 years), treatment with surgery or intrahepatic chemotherapy (100%), and longer disease free interval from melanoma primary tumor diagnosis to metastasis (mean 125.9 months, median 94 months). For comparison, the series by Rietschel et al. had a median 53 month disease free interval in their unselected series with a high proportion of long survivors [8].
In conclusion, further study of larger numbers of long-survivors and short-survivors with uveal melanoma is needed to identify biologic differences that could be utilized to promote longer survival and better management for patients with metastatic uveal melanoma.
ACKNOWLEDGEMENT
Funding: The Patti Blow Research Fund and OSU Department of Ophthalmology and Visual Sciences Research Fund.
CONFLICT OF INTEREST
Declared none.


