All published articles of this journal are available on ScienceDirect.
Ischemia Modified Albumin (IMA) as a New Biomarker in the Ophthalmology Field: A Brief Literature Review
Abstract
Purpose:
This study aimed to review the potential role of ischemia-modified albumin as a biomarker for diagnostic modalities in the ophthalmology field.
Methods:
Articles were reviewed without a specific date. A manual search was also performed by reviewing reference lists of meta-analyses and systematic reviews. All articles were reviewed, and a total of 18 articles were selected by the authors.
Results:
Oxidative stress increases structural and functional damage to proteins in many ocular diseases. The human serum albumin is a major circulating protein with antioxidative and anti-inflammatory properties. Oxidative stress has been shown to be an important part of etiology and pathogenesis in ocular diseases related to ischemia. Biomarkers that are specific to oxidative stress and ischemia-related ocular pathogenesis are needed to provide an extensive understanding regarding diagnosis, monitoring progression, and new potential target treatment. Ischemia-modified albumin (IMA) as a new promising biomarker might be useful in the early detection and treatment of ocular diseases with ischemic pathogenesis.
Conclusion:
IMA plays an important role in the progression of ophthalmology diseases, such as diabetic retinopathy, hypertensive retinopathy, cataract progression, seasonal allergies, and glaucoma. Further studies are needed to elaborate these results as a consideration in new testing modalities in clinical practice as well as a new target therapy research.
1. INTRODUCTION
According to WHO, there are 1 billion people with low vision or blindness, with half of the cases being preventable. Highly prevalent causes are refractive error, cataracts, glaucoma, corneal opacities, diabetic retinopathy, and trachoma. Age-related macular degeneration, cataract, diabetic retinopathy, and glaucoma are sight-threatening diseases with high prevalence in the United States [1, 2]. These potentially blinding diseases can be prevented and managed with good outcomes if diagnosed in the early stage. Due to the chronic progressivity of the diseases that may lead to blindness, new treatment approaches are needed.
Free radicals are produced either by internal normal cell metabolism or external factors, such as pollution, cigarette smoke, radiation, or medication. Increased formation of free radicals and decreased antioxidant defense mechanisms resulting in oxidative stress may play a role in the pathogenesis of chronic eye diseases [3]. Oxidative stress increases structural and functional damage to several protein structures. Human serum albumin is an important and abundant circulating protein with antioxidant and anti-inflammatory properties. In addition, oxidative stress has been shown to play an important role in the pathogenesis of chronic ocular diseases. Therefore, systemic and topical antioxidant treatment may be considered a new target for treatment [4-6].
Ischemia-modified albumin as a biomarker for diagnostic purposes was initially studied for myocardial ischemia cases in 1990. It has been more recognized nowadays in clinical settings [7]. Albumin plays an important role as the majority (more than 40%) and most variable protein in the bloodstream. The transitional metal-binding site N-terminus of albumin for metals, including cobalt, copper, and nickel, is prone to degradation and decrease due to structural changes in ischemia conditions. Ischemia-modified albumin is an isoform due to structural changes [8, 9]. Albumin has metal-binding properties that are altered during ischemic events, detected by ischemia-modified albumin (IMA). IMA has recently been suggested as a pathologic marker for ischemic pathogenesis diseases [10, 11]. Data regarding IMA as a novel and sensitive biomarker in ophthalmology is still limited. IMA can be useful for diseases with ischemia and oxidative stress as pathogenesis. Molecular biomarkers for early diagnosis should be reproducible, specific, and sensitive. Moreover, IMA serum levels are non-invasive testing that can be easily measured and have good reproducibility, but unfortunately, study regarding IMA as a novel and sensitive biomarker in the ophthalmology field is still limited [12]. This review aims to identify studies on IMA in ophthalmology, as well as future considerations for early diagnostic modalities and new target therapy.
2. METHODS
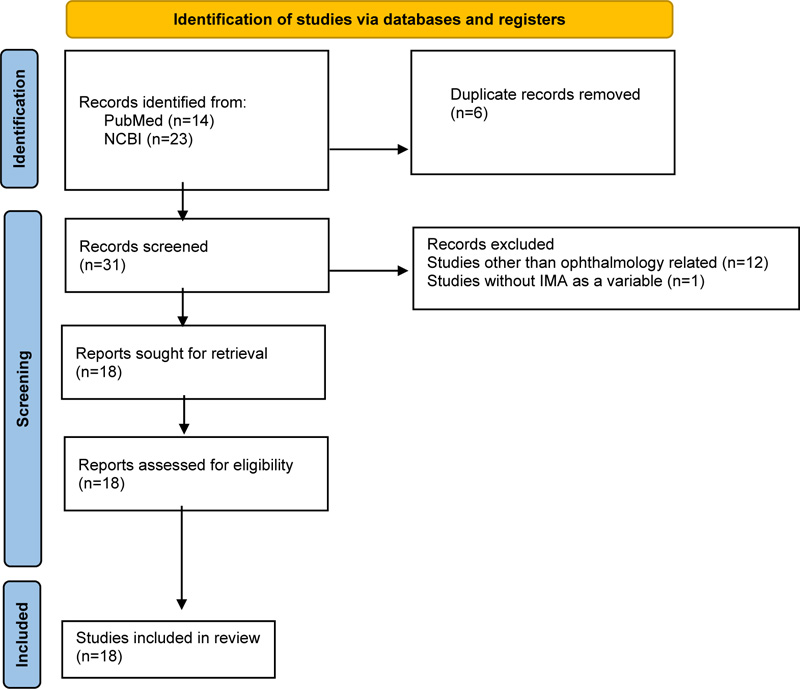
Studies were collected from four search databases, such as PubMed, NCBI, and Cochrane Library. There was no specific range of years selected during the search. The search terms used to select the relevant studies were “ischemia modified albumin” (Supplementary Concept) OR “ischemia modified albumin” (All Fields) OR “ischemia modified albumin” (All Fields) AND “ischemia modified albumin” (Supplementary Concept) OR “ischemia modified albumin” (All Fields) OR “ischemia modified albumin” (All Fields) AND “ophthalmology” (All Fields) OR “ophthalmology” (MeSH Terms) OR “ophthalmology” (All Fields) OR “ophthalmology” (All Fields). The inclusion criteria were research papers written in English, not letters or editorial papers. A manual search was also performed by reviewing reference lists of meta-analyses and systematic reviews. A total of 37 articles were found during the search, 14 articles found in PubMed, 23 articles found in NCBI, and none in Cochrane Library. Then, 6 duplicate articles were removed, with 13 articles excluded due to topics outside the ophthalmology field and 1 article without IMA as a variable. The authors reviewed a total of 18 articles. All articles were reviewed and selected by the authors. The screening was done by reviewing the abstract, and inclusion and exclusion were set to select the articles (Fig. 1).
3. IMA AS A DIAGNOSTIC MARKER
Albumin plays an important role and is one of the most variable proteins consisting of 40% in the bloodstream. Albumin synthesizes daily about 25% of the total proteins in the human body [14]. Albumin has a metal binding capacity in an ischemic state, which can change it into a transitional form. Human serum albumin consists of numerous peptides constructed by various amino acids. The transitional metal-binding site, i.e., the N-terminus, of albumin for metals, including cobalt, copper, and nickel, is prone to degradation and decrease due to structural changes in ischemia conditions. Ischemia-modified albumin becomes isoform due to structural changes. Several conditions that can modify serum albumin to IMA include hypoxia, acidosis, superoxide radical injury, energy-dependent membrane disruption, free iron, and copper exposure. Such conditions will cause metabolic alterations in the albumin due to posttranslational changes, including oxidation, glycation, carbamylation, nitrosylation, guanylation, dimerization, and truncation [4].
The metal binding properties of albumin are altered during ischemic events detected by ischemia-modified albumin (IMA). It has been recently suggested as a novel diagnostic marker with a short half-life for ischemic pathogenesis diseases [4, 15]. In general, IMA in a hypoxic state can increase within minutes and remain elevated for up to 12 hours. It is previously considered a sensitive biomarker for myocardial necrosis at the time of the acute ischemic attack [9, 10, 14]. IMA presence in serum will return to the baseline range 12-24h after the onset of an ischemic cardiac event. Other than cardiac diagnosis, ischemia that occurs in other ischemic events also cause changes in albumin; therefore, IMA may not be considered a specific marker. Other diseases can also be responsible for increasing levels of IMA, such as systemic sclerosis, peripheral vascular disease, skeletal muscle ischemia, cerebral ischemia, and diabetes mellitus [11, 12, 14]. IMA is most likely correlated with oxidative stress [16]. IMA also increases due to inflammation, ischemia, and decreased antioxidants in other organ systems due to ocular diseases [17, 18]. IMA is quantified with the albumin cobalt binding test (ACB) based on the structural changes in albumin induced by ischemic events measured with spectrophotometric assay [8].
3.1. IMA and Diabetic Retinopathy
Diabetes mellitus (DM), according to World Health Organization (WHO), is an epidemic worldwide and will be the seventh leading cause of death in 2030. Based on the epidemiology study conducted by WHO, the prevalence of diabetes for all ages worldwide was estimated to be 171 million (2.8%) in 2000 and will increase to as many as 366 million (4.4%) in 2030 [19]. Diabetes mellitus (DM) can cause several complications, such as microvascular and macrovascular complications. Ocular complications, such as retinopathy, papillopathy, cataract, glaucoma, and ocular surface disease, can cause morbidity and decrease quality of life. Complications can be prevented by early diagnosis and treatment. The most common cause of blindness is diabetic retinopathy (DRP) [20]. Endothelial damage may occur as a result of prolonged exposure to biochemical and physiological changes in the hyperglycemia state, leading to macular edema, which causes visual impairment in diabetic patients [21].
Retinal ischemia and the development of neovascularization of the retina are the pathogenesis developed from abnormalities in glucose metabolism, hyperglycemia, and oxidative stress found in diabetes [22]. Diabetic retinopathy (DRP) is microangiopathy developed from increased vascular permeability, ocular hemorrhage, and lipid exudate and is also characterized by the formation of new vessels on the retina and the posterior vitreous surface. The microangiopathy condition has a slow and progressive change that leads to blindness [20], [23]. Previous studies have been able to evaluate the development of DRP, diabetes, blood glucose level, and hemoglobin A1c (HbA1c) but failed to associate them with tissue ischemia [22, 24]. With the increasing prevalence of diabetic retinopathy and the lack of biomarker testing modalities that contribute to earlier diagnosis, finding new diagnosis markers with good reproducibility and low cost would be a possible solution to halt the development of DRP that currently has no effective treatment [25, 26].
Pathogenesis of diabetes associated with oxidative stress (OXS) has been proposed to have an association with retinal damage in DRP. OXS can damage protein structure and function. Concurrently, albumin is a major protein circulating in the human body. Albumin with modified structures and forms known as ischemia-modified albumin (IMA) may increase, especially in diseases with ischemia-based pathogenesis. An increase in IMA levels has been suggested as a marker to measure elevated OXS in diabetes. However, data regarding IMA in DRP are still limited [25-27]. In contrast to increased OXS in diabetes, antioxidant defense systems, such as glutathione (GSH), decrease in DM [28]. The first research on IMA levels in diabetes by Piwowar et al. stated that IMA levels were higher in the diabetes group compared to the control group [14]. Referring to a meta-analysis done by Reddy et al., based on five studies, IMA is a simple biomarker that has the ability to monitor OXS levels in DRP and practically predicts progression in DRP. They included medium to high-quality, hospital-based case-control or observational studies including diabetic patients with or without DRP compared to the healthy control group. A comparison of serum IMA levels in DR and control subjects demonstrated significant heterogeneity. In addition, another finding showed that serum IMA levels in DRP compared to DM subjects revealed a significant increase in DRP, and with sensitivity analysis, the results were also consistent. A case-control study by Turk et al. showed a significant positive association between the duration of disease and IMA. They were the first to conduct research on serum IMA levels in DRP cases. Based on the research, it can be concluded that IMA is a sensitive and specific new biomarker that depicts an overview of DRP. This result is also supported by Kalayci et al., demonstrating IMA as a novel biomarker for the early detection of micro-vascular hypoxia [29]. A study done by Turk et al. has limitations regarding the inability to compare serum IMA levels between non-proliferative and proliferative diabetic retinopathy [24]. Another research by Jia et al. concluded a significantly higher level of serum IMA in proliferative DRP compared to non-proliferative DRP. IMA has been proposed to be a significant risk factor for DRP progression [30]. This finding is also similar to the research conducted by D’Souza et al., who found significantly higher IMA serum levels in type 2 DM compared to control subjects. Moreover, higher levels of IMA in diabetic groups with complications were revealed compared to those without complications [31]. Similar results were found in a study done by Kirboja et al., which concluded that IMA serum levels were statistically significant as a risk factor for developing diabetic retinopathy (P = 0.003) [32]. Concerning the role of oxidative stress as pathogenesis in diabetes, a study by Reddy et al. aimed to find a correlation between IMA and GSH as an antioxidant defence mechanism in DM and diabetic retinopathy patients. Hyperglycemia conditions in diabetes lead to oxidative stress and elevation of IMA levels. This condition is considered to initiate glycosylation of hemoglobin process that increases the affinity to oxygen and an increased reluctance to release oxygen, inducing tissue hypoxia and oxidative stress. Based on this study, both hyperglycemia and an increase in OXS are associated with a decrease in GSH. Hyperglycemia could be evaluated by fasting plasma glucose (FPG) levels and oxidative stress by IMA levels. The condition where both FPG and IMA levels are elevated is related to the lowered antioxidant profile reflected by GSH. Diabetic retinopathy subjects have high OXS with increased IMA and decreased GSH (P<0.005). Moreover, FPG levels and HbA1C are found to be positively associated with IMA. Negative correlations are established between FPG (r = -.052, P = 0.02) and IMA (r = - 0.49, P = 0.03) [33]. Kalayci et al. found lower IMA serum levels in proliferative DRP compared to non-preliverative DRP, which might occured as the body’s compensation mechanism to ischemia process. Furthermore, higher IMA levels also correlated with macular edema in diabetic groups [29].
Even though data regarding the mechanisms of oxidative stress-inducing diabetic complications are still limited, this process tends to increase in diabetes. As a result, reactive oxygen species increase and impair the retinal microvascular network. Oxidative stress leads to blood-retina barrier disturbances and impairment of retinal microvascular blood flow, resulting in capillary occlusion, perfusion reduction, ischemia, and neovascularization. Progressive perfusion reduction may lead to cotton wool spots, venous beading, intra-retinal microvascular abnormalities, and neovascularization. Retinal capillary changes include occlusion and thickening of basement membrane resulting in retinal non-perfusion and decompensation of endothelial barrier function, leading to macular edema. IMA is considered a novel, highly reproducible, and low-cost test that can further determine microvascular ischemic OXS in the early stage to prevent retinal complications. IMA is also associated with identifying the duration of increased glucose levels and glycosylated haemoglobin [24, 25, 29, 33, 34].
3.2. IMA and Hypertensive Retinopathy
WHO estimates 1.28 billion people aged 30-79 with hypertension worldwide, with the highest prevalence of up to two-thirds in low and middle-income countries. Uncontrolled hypertension can lead to severe complications causing morbidity, affecting the cardiovascular, renal, cerebrovascular, and ocular systems in the form of hypertensive retinopathy. Detection of hypertension retinopathy in an early stage has been proposed by other researchers by using biochemical markers that are related to inflammation, endothelial cell activation, oxidative stress, and angiogenesis. Hypertension itself is related to the oxidative stress mechanism. IMA is a novel biomarker with nonspecific properties regarding tissue ischemia and oxidative stress [35, 36]. Pavlovschi et al. compared IMA serum levels in the tears of the hypertension group and control group. The hypertension group consisted of different grades based on their severity, such as grade I, grade II, and grade III. They found a higher level of IMA in the hypertension group, and their level increased in accordance with the severity of the disease. Serum levels of IMA were found to be superior in detecting hypertension retinopathy compared to tear samples. This finding suggests that hypertensive retinopathy is correlated with oxidative stress mechanisms [37].
3.3. IMA and Seasonal Allergic Conjunctivitis
Seasonal allergic conjunctivitis (SAC) is the most common ocular allergy and affects 6-30% of the general population worldwide [38]. Cell homeostasis in the human body has a mechanism of oxidant generation. This process leads to the release of mediators, including reactive oxygen species. Oxidative stress is defined as an imbalance between prooxidants and antioxidants in the body. Oxidative stress responses have been proposed as one of the pathogenesis of allergies [39]. Mast cells also play an important role as cell responses to allergies. Antigen exposure to the human body can lead to a sensitization process inducing degranulation of mast cells and release of mediators. In several studies, it was found that oxidative stress may affect mast cell degranulation [38], [40]. Oxidants are able to modify protein structures, such as albumin. As mentioned before, IMA is a sensitive and novel marker for evaluating oxidative stress [14]. Dadaci et al. concluded that IMA serum levels were found to be significantly higher in patients with SAC, which may be related to the role of oxidative stress in the pathogenesis of allergies. Increased IMA serum level is an indicator of an oxidative stress process. This condition may aggravate the allergic reaction. Therefore, IMA is suggested to have a strong relationship with oxidative stress in seasonal allergic conjunctivitis [41].
3.4. IMA and Cataract Progression
Cataracts are the leading cause of preventable blindness worldwide, with prevalence increasing from 12.3 million in 1990 to 20 million in 2020. The prevalence of blindness with cataracts as an etiology of blindness in Southeast Asia is 42%. It is considered higher than in high-income countries, such as North America [42, 43]. The development of cataracts occurs as a result of increasing ocular oxidative stress. These stress states occur when reactive oxygen species (ROS) are higher than antioxidant defense, leading to the denaturation of intracellular molecules, such as nucleic acids, proteins, and lipids. ROS are mostly produced in the lens, epithelium cells, superficial fiber cells, and aqueous humor. When the concentration level is high, ROS are considered toxic. Moreover, ROS can lead to lipid peroxidation of polyunsaturated fatty acids and insolubilization of crystalline lens [43, 44]. Based on this pathogenesis, a study was done by Elmazar et al. comparing IMA levels in cataract patients compared to control groups to evaluate IMA as a marker for oxidative stress and identify its relationship with cataract progression. IMA levels were found significantly higher in cataract patients than in control groups. Besides, IMA levels also showed significant differences between cataract types, hence the different levels of oxidative stress. IMA levels were found higher in the cortical cataract group [45].
4. IMA AND GLAUCOMA
Glaucoma accounts for 12% of the causes of blindness in the world. WHO Vision 2020 focuses on the prevention and management of blindness [46]. Adequate management for glaucoma cases needs to be done to achieve a more favourable outcome. However, diagnostic rates in Indonesia are still considerably low. Based on the Indonesian Healthcare Ministry (Kemenkes, 2015), the prevalence of PACG vs. POAG was 51.4% and 41.4%, respectively. Most of these cases were diagnosed in the late stage, when 13.5% were already blind [47, 48]. Glaucoma is a chronic progressive neuropathy that represents a group of diseases defined as a characteristic optic neuropathy associated with remodelling of the connective tissues of the optic nerve head and loss of neural tissue, presenting with the eventual development of distinctive patterns of visual dysfunction [49].
Biomarkers for glaucoma disease could be beneficial as indicators of the disease’s pathogenesis in either the early stage of the disease or management, but data regarding glaucoma biomarkers are limited [50]. In glaucoma cases, the nerve fiber layer has various stages of defects. An objective examination of these processes is beneficial but difficult to obtain in a clinical setting. Other possible structures that can be routinely tested include aqueous humor, vitreous body, tear film, and blood serum. Aqueous humor consists of proteins from the anterior segment and supplies nutrients, distributes signaling molecules, and removes metabolic waste. Ganglion cell death induced by apoptosis in glaucoma may alter the composition of proteins and metabolites. However, extracting aqueous humor for testing purposes in a clinical setting is invasive. Therefore, many researchers studied how to identify molecular markers that can detect the pathogenesis of glaucoma disease in a non-invasive manner. Novel glaucoma biomarkers could be advantageous for early diagnosis, disease progression, and evaluation of treatment responses [22, 51].
Glaucoma has been associated with lower levels of antioxidant defence mechanisms. Compromised local blood flow and vascular insufficiency lead to hypoxia in retinal ganglion cells [7, 52-54]. Glaucoma pathogenesis consists of the acceleration of apoptosis induced by the ischemia event. This process may result in an elevation of the IMA level. However, data regarding the role of IMA in ophthalmology are still limited. Based on a meta-analysis of oxidative and antioxidative markers in chronic glaucoma, IMA is proposed as a new marker of oxidative stress [54]. Karakurt et al. found that elevated levels of IMA and adjusted IMA correlate with vascular insufficiency and oxidative stress. In other studies, visual field defect progression was found to be associated with the level of oxidative stress [4]. Gulpamuk et al. revealed higher IMA levels in POAG compared to OHT and the controlled group [55].
These findings were similar to research done by Chang et al. by comparing IMA serum levels in primary angle-closure glaucoma (PACG) and control subjects. They found that serum IMA levels were significantly elevated in PACG. The study also showed that IMA level and elevation were associated with acute onset of ischemia in relation to oxidative damage. They proposed IMA as a new biomarker of oxidative stress in PACG [8, 55]. Another study by Comez et al. demonstrated similar results to Chang et al. regarding the highest elevation of IMA in glaucoma patients. Hence, IMA is considered a novel marker for the acute onset of ischemic events. Comez et al. also compared the level of IMA in serum and aqueous humor in the rabbit eye and showed a lower IMA level in aqueous humor. Up to this point, no research involving human subjects has been done due to the invasive nature of obtaining aqueous humor [56].
CONCLUSION
IMA is considered a novel biomarker in ophthalmology based on the ischemia pathogenesis of the related disease. Previous studies have shown a correlation between IMA with diabetic retinopathy, hypertensive retinopathy, seasonal allergic conjunctivitis, cataract progression, and glaucoma, although data are still limited. IMA has a good reproducibility testing value and is a non-invasive method that has significant potential to improve the diagnosis and treatment of various potentially blinding ocular diseases. Further studies are needed to elaborate on these results as a consideration in testing modalities in clinical practice in conjunction with future research on new target therapy.
LIST OF ABBREVIATIONS
| IMA | = Ischemia-Modified Albumin |
| DM | = Diabetes Mellitus |
| WHO | = World Health Organization |
| ACB | = Albumin Cobalt Binding Test |
| DRP | = Diabetic Retinopathy |
| FPG | = Fasting Plasma Glucose |
| ROS | = Reactive Oxygen Species |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
Declared none.


