All published articles of this journal are available on ScienceDirect.
Supraciliary Hema Implant in Combined Deep Sclerectomy and Phacoemulsification: One Year Results
Abstract
We present the combined surgery of non-penetrating deep sclerectomy with insertion of the implant in the supraciliary space as an effective and safe surgery for patients with both cataract and primary open angle glaucoma. This study included 20 eyes of 16 patients who were followed up during 12 months. We found a significant intraocular pressure reduction, changing from a preoperative mean of 23 ± 5 mmHg to a postoperative mean of 18 ± 3 mmHg (p<0.002). Similarly, a significant reduction in the number of glaucoma drugs needed was observed, varying from 2.5 ± 0.9 drops per patient to 0.7 ± 0.9 (p<0.0002) at the end of the study. We also report a significant improvement in best-corrected visual acuity, from 5/10 ± 2/10 to 8/10 ± 2/10 (p<0.006), one year after the combined surgery. The only intraoperative complication observed was the microperforation of the trabeculo-descemetic membrane (TDM) and postoperative complications were iris incarceration, seidel test positivity and microhyphema. All these complications resolved successfully.
INTRODUCTION
The combination of phacoemulsification and non-penetrating deep sclerectomy (phaco-NPDS) has been used for the surgical treatment of cataract and open angle glaucoma (OAG) since the first reports in the late 90s [1,2]. It has proved its efficacy and safety when compared with other surgical techniques, such as phacoemulsification combined with trabeculectomy [3,4].
Deep sclerectomy is a non-penetrating procedure for the treatment of OAG that can be enhanced with the use of antimetabolites, such as mitomycin C (MMC) or 5- fluorouracil, as well as with implants. These devices are placed to facilitate the aqueous outflow, by maintaining the virtual space created after removing the deep scleral flap. There is proof of the good long-term outcomes of its implantation in the scleral bed [5,6], but there is only one report of the placement of the implant in the supraciliary space [7]. The present study is the first report showing the outcomes of deep sclerectomy with supraciliary implant combined with cataract surgery.
MATERIALS AND METHODOLOGY
Twenty-three eyes of 19 patients with significant cataract and medically uncontrolled OAG, were included in this prospective study. Exclusion criteria were age less than 50 years, African-American race, concurrent ocular inflammatory diseases, previous ocular surgeries, brunescent cataract or angle synechia of 180º degrees or more. Twenty eyes of 16 patients completed the 12 months follow up and were considered statistical purposes. The other three patients did not finish the study for several reasons: one patient failed to attend the appointments; another developed an advanced degree of senile dementia during the follow up; and the third patient had to be excluded from the study due to intraoperative microperforation followed by a postoperative irreducible iris incarceration that needed early reintervention changing to a trabeculectomy. We did not consider these three patients in the outcome data, but we included them in safety analyses.
Medically uncontrolled OAG was defined as an intraocular pressure (IOP) higher than 21 mmHg under a maximal tolerable therapy with evidence of progression of visual field defects and/or optic nerve cupping.
Demographics of the sample are shown in Table 1. In the preoperative assessment, patients underwent a complete examination including best-corrected visual acuity (BCVA) in Snellen decimal scale, biomicroscopy, Goldmann tonometry, gonioscopy, funduscopy. We performed a corneal pakimetry using an ultrasonic pakimeter (UP-1000®, Nidek, Tokyo, Japan) and an ultrasonic immersion biometry for the intraocular lens (IOL) calculation (Ocuscan®, Alcon, Fort Worth, TX, USA). All patients also underwent an automated perimetry (Humphrey 740i, Carl Zeiss Meditec, Dublin, CA, USA), staging their visual field damage as mild, moderate or advanced defect according to Hodapp, Parish and Arderson classification [8].
Demographics of the Sample
| N | Mean | SD | Range | |
|---|---|---|---|---|
Sex
|
5 (31.3%) 11 (69.7%) |
|||
Eye
|
8 (40%) 12 (60%) |
|||
Type of glaucoma
|
16 (80%) 2 (10%) 2 (10%) |
|||
Campimetric stage
|
15 (75%) 3 (15%) 2 (10%) |
|||
| Age (years) | 76.35 | 6.88 | 61-89 | |
| VA (Snellen decimal chart) | 5/10 | 2/10 | 1/10-10/10 | |
| IOP (mmHg) | 22.80 | 4.93 | 16-36 | |
| Number of medications | 2.55 | 0.94 | 0-4 | |
| Pakimetry (µm) | 556.6 | 44.47 | 490-630 |
SD: standard deviation, POAG: primary open angle glaucoma, PSXG: pseudoexfoliative glaucoma, PG: pigmentary glaucoma.
Surgical Technique
All surgeries were performed, under retrobulbar anaesthesia and intravenous sedation, by the same surgeon (JL). The surgical procedure included a standard phacoemulsification, intraocular lens (IOL) insertion in the capsular bag (Monobloc IOL Y601075, AJL Ophthalmics, Álava, Spain) and a corneal suture. After the cataract surgery, a deep sclerectomy was performed following the technique firstly described by Mermoud [9] with the use of MMC. The hema implant (V-2000, Esnoper®, AJL Ophthalmics, Álava, Spain) (Fig. 1) was placed in a full thickness scleral pocket, made with a 45º blade for the incision and a blunt spatula, 2mm behind the scleral spur and sutured with a 10/0 nylon suture (Fig. 2).

Hema implant model. (V-2000, Esnoper®, AJL Ophthalmics, Álava, Spain).
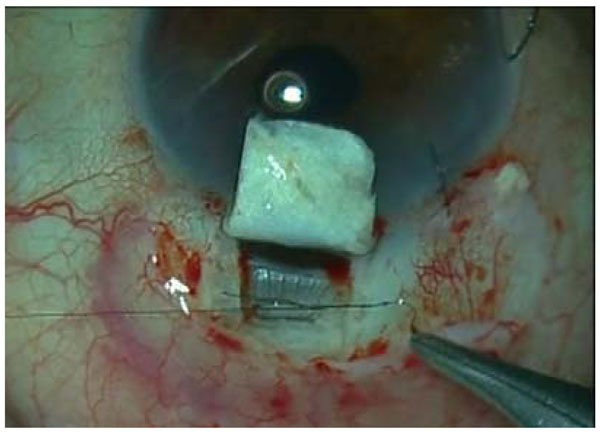
An intraoperatory image of the implant placement in the supraciliary space while being sutured.
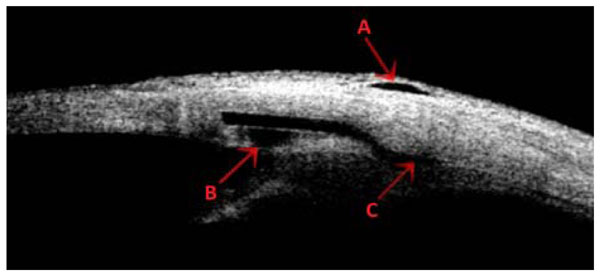
Ultrasound biomicroscopy of a bleb. The red arrows show the subconjunctival (A), intraescleral (B) and supraciliary (C) spaces for aqueous drainage.
Follow up visits were scheduled at one day, one month, 3 months, 6 months and 12 months after the surgery and all visits included BCVA and IOP measurement. The need for glaucoma treatment or the performance of a goniopuncture were recorded. Topical glaucoma medication was started if the IOP was higher than 21mmHg or there were signs of glaucoma progression during the follow up. A goniopuncture was considered when IOP was higher than 12 mmHg. Only the 12 months results were analysed for statistical purposes.
Complete success was defined as IOP less or equal to 21 mmHg without additional glaucoma medication and qualified success as IOP less or equal to 21 mmHg with or without additional glaucoma medication. The outcome was considered a failure when IOP was lower than 6 mmHg or greater than 21 mmHg despite the use of glaucoma drops, the eye required further glaucoma surgery or lost perception of light [10].
Statistical Analysis
The quantitative variables were IOP, number of glaucoma drugs used and BCVA, before and after the surgery. This data is presented as mean and standard deviation. We used the d’Agostino-Pearson normality test to assess the symmetry and kurtosis of the variables. The two tails Wilcoxon test for paired data with a 95% confidence interval was used for the statistical analysis of the sample, and a p value of 0.05 or less was considered as significant.
RESULTS
Mean preoperative IOP was 22.8 ± 4.9 mmHg in our sample. Twelve months after the surgery we observed an statistically significant IOP reduction, with a mean of 18 ± 3.1 mmHg after this period (p<0.002).
There was also an statistically significant reduction in the number of topical treatments after the surgery, starting with a mean of 2.5 ± 0.9 drops per patient and decreasing to 0.7 ± 0.9 at the end of the study (p<0.0002). BCVA improved from 5/10 ± 2/10 to 8/10 ± 2/10 (p<0.006) one year after the surgery. Complete success was achieved in 70% of the patients and qualified success in 90% of them, with a failure rate of 10%, 6 months postoperatively. Twelve months after the surgery, complete success was 45%, qualified success was 95% and the failure rate 5%.
At the end of the study, 80% of the patients had undergone Nd:Yag goniopuncture, with a mean time between the surgery and the procedure of 77.4± 63.4 days. No complications related to goniopuncture were recorded.
For the safety analysis we considered the 23 eyes that underwent surgery. The only intraoperative complication was the microperforation of the trabeculodescemetic membrane (TDM), observed in four patients. There were no complications related to the phacoemulsification procedure. Postoperative complications included microhyphema in three patients and seidel test positivity (lack of watertightness of the bleb) in two patients 24 hours after the surgery. Both seidel test positivity cases resolved spontaneously one week after the surgery. Iris incarceration in the trabeculo-descemetic membrane (TDM) was observed in two patients, and it was successfully managed with green-laser iridoplasty and Nd:Yag-laser lysis of the synechia in one of them. The other case was irreducible and had to be reconverted into a trabeculectomy.
DISCUSSION
The surgical management of patients with two concurrent ophthalmic pathologies can be controversial. In cases of glaucoma associated with cataract, the approach will depend on the type of glaucoma and its stage, the visual requirements of the patient as well as the surgical experience of the surgeon [11]. The efficacy and safety of phaco-NPDS, a surgical option for these patients, has been shown in several studies [1,2,6,12-16]. This surgery can be enhanced with the use of MMC or an implant placement. Both procedures have shown its efficacy to potentiate the IOP reduction [6,17,18]. We have used a supraciliary hema implant in order to improve the uveoscleral outflow of aqueous humour through the floor of the implant by maintaining the intraescleral and subconjunctival outflow paths [7]. This three filtering spaces can be visualized on the ultrasound biomicroscopy (Fig. 3).
In our study, we have analysed 20 eyes after phaco-NPDS and we have found a 21% reduction in IOP levels, 12 months after the surgery. According to the classical definition in glaucoma surgery, the complete success rate in our sample is 45% and the qualified success is 95% at the end of the follow up. Only one patient was considered as a failure for having an IOP of 22 mmHg under maximal topical medication. Other authors have published similar results: Gianoli [1] reports a 59% complete success rate and Di Staso [2] finds a 73.3% success rate, not distinguishing between complete and qualified success. Muñoz-Negrete describes a 67.6% complete success in a study with 39 patients [12], meanwhile Moreno-López reaches an 80% complete success without the use of any implant, in a study with 15 eyes of 12 patients [15]. None of these authors have used intraoperative MMC in their studies.
There is only one publication showing the results of NPDS using a supraciliary implant [7]. It is difficult to compare our results with this study, because they evaluated NPDS alone without cataract extraction, and the implant used was different to ours (T-flux. IOLTECH Laboratoires, La Rochelle, France). Nevertheless, he reports an overall success rate of 93.4% similar to the 95% of our study.
Although we have used the widely known success rate to evaluate our outcomes and compare them to other studies, it is uncertain whether it is a useful tool to validate a glaucoma surgical technique. Well-recognized cohort studies such as the Advanced Glaucoma Intervention Study [19], the Collaborative Initial Glaucoma Treatment Study [20] or the Early Manifest Glaucoma Trial [21] have proven that lowering IOP correlates well with slower visual field defects progression and recommend a lower target IOP in patients with more evolved disease. This is the reason why target IOP should be individualized.
We have found a significant reduction in the number of topical drugs used by our patients, changing from a mean of 2.5 ± 0.9 before the surgery to 0.7 ± 0.9 at the end of the follow up. These results are similar to that of Gianoli (0.6 ± 0.8) [1] and higher than the 0.34 ± 0.6 of Muñoz-Negrete [12] or the 0.3 ± 0.2 described by Muñoz [7].
The rate of Nd:Yag goniopuncture (80%) in our group is higher than other studies [22,23]. This could be because we understand goniopuncture almost as a part of the NPDS procedure, and not as a solution to a postoperative complication. In our hands it enhances aqueous humour drainage and helps to maintain the uveoscleral, intrascleral and subconjunctival spaces created with the surgery.
The most common intraoperative complication was trabeculo-descemetic membrane(TDM) microperforation, occurring in 17.4% of our patients. This is slightly higher to what has been previously published by Funnell et al. (15.7%) and Anand et al. (14.45%) [14,18]. This situation is related to a higher incidence of postoperative complications and this is why some authors have evaluated these results independently [14].
The limitations of this study are multiple, but are mainly the small sample size and the lack of a control group. The question whether placing an implant in the supraciliary space produces a higher reduction in IOP than in the scleral space, is a question that cannot be answered with our study, and would require a bigger sample size study comparing both techniques.
CONCLUSION
Phaco-NPDS with supraciliary implant provides a significant reduction both in IOP and glaucoma topical medication, with minor complications that tend to resolve. This combined surgery allows a fast visual rehabilitation and avoids the discomfort that two separate surgeries represent to the patient.
ACKNOWLEDGEMENT
AJL Ophthalmics has helped to fund this article.
CONFLICT OF INTEREST
The authors have no proprietary interest in any of the materials described in this article.


